This state of matter is shown in the image below:
What is a solid?
(DOUBLE JEOPARDY!!) These are the Five (5) Flags of a Chemical Change.
Color Change, Gas Production (Bubbles), Heat Gain/Loss, Light Emission, Precipitate Formation
(TRIPLE JEOPARDY!!!) This is one of the elements that demonstrate very little reactivity with any other element on the Periodic Table, and it exists as a gas at room temperature.
What is Helium, Neon, Argon, Krypton, Xenon, Radon, or Oganesson?
(DOUBLE JEOPARDY!!) This term describes the tendency of an atom to attract electrons to itself.
What is electronegativity?
This scientist's concept of the atom closely mirrors the concept imagined by the Greek philosopher Democritus.
Who is John Dalton?
(TRIPLE JEOPARDY!!!) This is the part of the spectrum that humans can see.
What is VISIBLE LIGHT?
(DOUBLE JEOPARDY!!) This term describes atoms of the same element with differing numbers of neutrons.
What is an ISOTOPE?
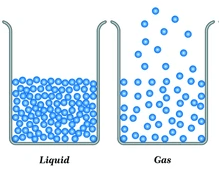
This state of matter can assume the shape of its container.
What is a liquid/gas?
This type of change occurs when you cut a sheet of paper with scissors.
What is a physical change?
This chemical family group includes elements with varying numbers of valence electrons and high melting points.
What is the Transition (Heavy) Metals group?
This element is the most electronegative element on the Periodic Table.
What is Fluorine?
This model places electrons on specific, discrete energy levels orbiting the nucleus
What is the Bohr Model?
This is the mathematical relationship between frequency and wavelength.
What is an INVERSE RELATIONSHIP?
This is the number of neutrons in the isotope below:
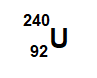
148 NEUTRONS
(DOUBLE JEOPARDY!) This state of matter features particles vibrating in fixed positions, usually in a pattern.
What is a solid?
(TRIPLE JEOPARDY!!!) This type of physical property includes color, state, melting point, and density.
What is an intensive property?
(DOUBLE JEOPARDY!!) This is the most-reactive group of metals on the Periodic Table.
What is the Alkali Metals group?
This periodic trend increases as you go left to right across a period, and as you go up a chemical family.
What is electronegativity or Ionization Energy?
This subatomic particle was discovered by J.J. Thomson during his cathode ray tube experiment.
What is the electron?
This dangerous type of radiation has the highest frequency and shortest wavelength on the EM Spectrum.
What is GAMMA RADIATION?
(QUADRUPLE JEOPARDY!!!!) Explain the difference(s) between "AVERAGE", "MASS NUMBER", and "AVERAGE ATOMIC MASS".
An AVERAGE is taken by adding values together and dividing by the number of values added.
MASS NUMBER is the combined number of protons and neutrons in an atom.
AVERAGE ATOMIC MASS is the weighted average of all isotopes of an atom based on an isotope's abundance.
*SPECIAL TASK* Each team must choose their TALLEST team member for this special task:
(30 seconds) Draw a particle view of a liquid transitioning to a gas!
This type of property describes the conductivity, malleability, and luster of metals.
What is a physical property?
The element featured in the image below is most likely in this chemical family group: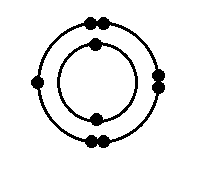
What is the Halogens (Halides) group?
(TRIPLE JEOPARDY!!!) Fill in the blanks: The atomic radii of atoms increases moving down a group in the periodic table because of additional _________ outside the nucleus, as well as ________ among electrons.
Electron Shielding/Energy Levels
Repulsion/Repelling
(DOUBLE JEOPARDY!!) Which postulates in Dalton's Atomic Theory are no longer correct?
Atoms are indivisible.
All atoms of the same element are identical.
Waves with the highest amounts of energy tend to have this level of frequency.
What is HIGHEST FREQUENCY?
(DOUBLE JEOPARDY!!) Calculate the AVERAGE ATOMIC MASS of Magnesium using the data below:
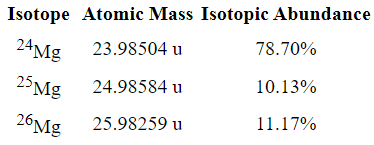
24.31 u
(TRIPLE JEOPARDY!!!) Briefly explain why solid H2O (ice) floats on top of liquid H2O (water).

Due to hydrogen bonding, molecules of solid H2O are spaced farther apart than molecules of liquid H2O. As a result, ice is less dense than water. Ice floats on water.
(QUADRUPLE JEOPARDY!!!!) A student cuts an iron pipe into two pieces, then places one outside for a few days until rust begins to appear. The other piece is heated until it melts.
Identify the type of changes occurring.
Chemical Change(s): rust formation
*SPECIAL TASK* Each team must choose their team member with a BIRTHDAY closest to today for this special task:
(30 seconds) Draw a Bohr Model for an Alkaline Earth Metal in Period 3.
This is the correct order - from least electronegative to most electronegative - for the elements Barium, Oxygen, Fluorine, and Strontium.
(TRIPLE JEOPARDY!!!) What did Ernest Rutherford observe during his Gold Foil Experiments that led him to believe atoms were mostly empty space?
He fired positively-charged alpha particles towards atoms of gold, to which most particles passed through the atoms.
*SPECIAL TASK* Each team must choose their YOUNGEST team member for this special task:
(30 seconds) Write the acronym that represents the VISIBLE LIGHT range in order from longest to shortest wavelength.
ROY G BIV
(DOUBLE JEOPARDY!!) Lithium has an AVERAGE ATOMIC MASS of 6.941 u and has two naturally occurring isotopes: 6Li and 7Li. Identify the isotope that is MOST ABUNDANT and explain why ...
7Li is the most-abundant isotope since the average atomic mass has a value significantly closer to 7 instead of 6.