What is the electron configuration for Li?
What is 1s2 2s1
What four letters represent the orbitals?
What is s, p, d, f
What is the electron configuration of Cl?
What is [Ne] 3s2 3p5
Complete the sentence:
Isoelectric atoms have the same number of ________
What is an Electron
In order to fill the 2s orbital, what orbital must be filled in first?
What is the 1s orbital
What is the electron configuration of F?
What is 1s2 2s2 2p5
How many electrons can an s orbital have?
What is 2
What is the electron configuration of Ca?
What is [Ar] 4s2
Which Noble Gas will Cs try to become?
What is Xenon
Out of these four orbitals, which one has the most energy: 1p 2d 3d 4s?
What is the 3d orbital
What is the electron configuration of Al?
What is 1s2 2s2 2p6 3s2 3p1
What is the element is represented with the following orbital notation?

What is Carbon (C)
What is the electron configuration of Zn?
What is [Ar] 4s2 3d10
What is happening to the electrons of Se in order to become like Kr?
What is gain 2 electrons
Which Principle/Rule states the following?
The orbitals are filled from the lowest energy level to the highest energy level.
What is Aufbau's Principle
What is the electron configuration of Ti?
What is 1s2 2s2 2p6 3s2 3p6 4s2 3d2
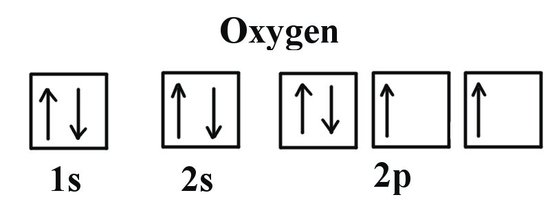
Write the orbital notation for Oxygen
What is the electron configuration of Ag?
What is [Kr] 5s24d9
What does "Iso-" mean?
What is equal to or same as
In order to fill the 4f orbital, what orbital must be filled in first?
What is is 6s orbital
What is the element that has this configuration: [Xe] 6s2 4f14 5d9?
What is Au
Write the Orbital notation for S
What is the electron configuration of Ba?
What is [Xe] 6s2
What is the Noble Gas Configuration for the following electron configuration?
1s² 2s² 2p⁶ 3s² 3p⁶ 4s² 3d¹⁰ 4p⁵
What is... [Ar] 4s²3d¹⁰4p⁵
!!DAILY DOUBLE!!
The Pauli exclusion principle states:
That only 1 electrons with the same spin (arrows) can occupy a single orbital
TRUE or FALSE
What is False