There are how many electron energy levels?
7
1: The energy level
s: The type of sublevel
2: The number of electrons
What does each box on an orbital diagram represent?
An orbital
Noble Gas Notation is done using which noble gas in relation the element being written?
The most recent previous noble gas
The Rules of Aufbau state that a level's p orbital will only filled once its ____ orbital has been completely filled first.
s
s: 2, p: 6, d: 10, f: 14
Every element after Argon will always be filled through which orbital?
3p
A line of 5 boxes linked together represents which type of orbital?
A d orbital
Which noble gas would be used to write the notation for Copper?
Argon
Opposite Spins
Which orbital is filled after the 4s orbital?
3d
What element has the following configuration?
1s2 2s2 2p6 3s2 3p6 4s2 3d7
Cobalt
What element does the below orbital diagram correspond to?
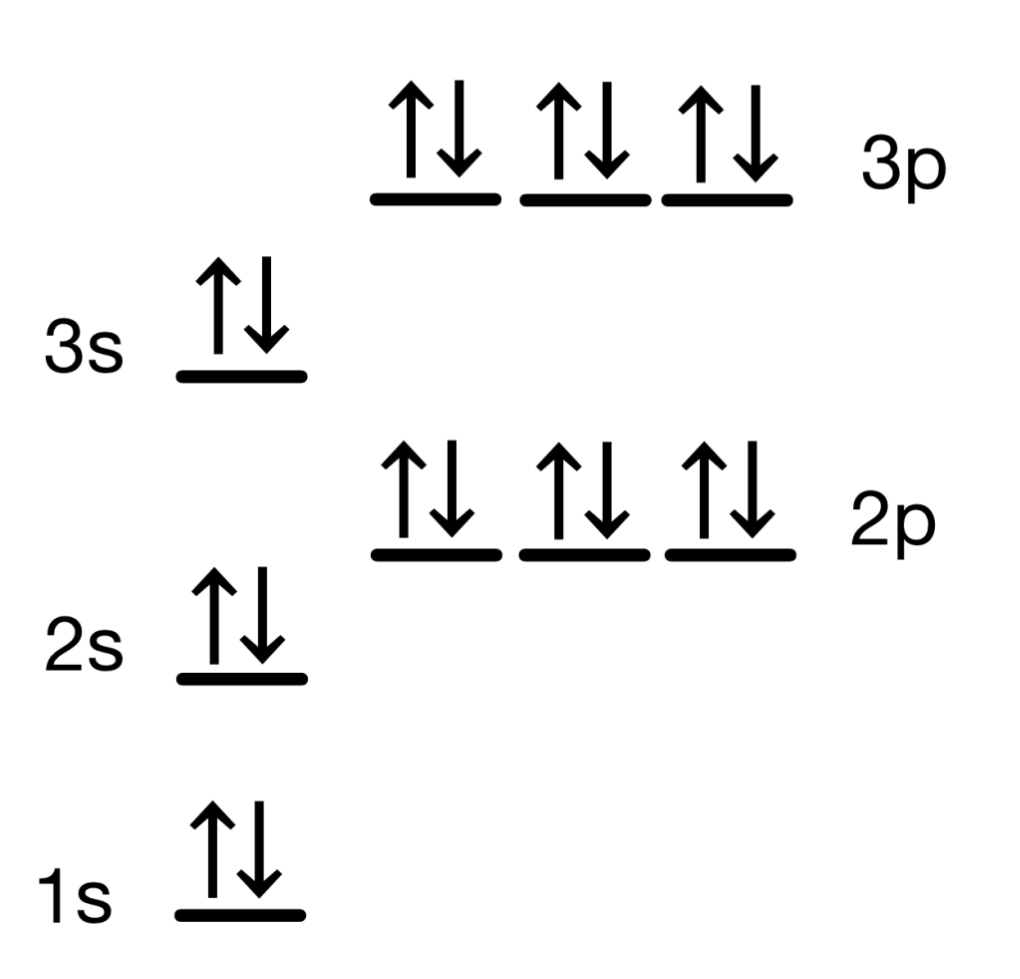
Argon
Which element has the following noble gas notation?
[Kr] 5s2 4d9
Silver
Hund's Rule states that all orbitals in a sublevel must have at least _____________ (Hint: bottom bunks)
One electron
What is the electron configuration for Niobium?
1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d3
Both of the following orbitals are incorrectly filled. Explain why BOTH are wrong.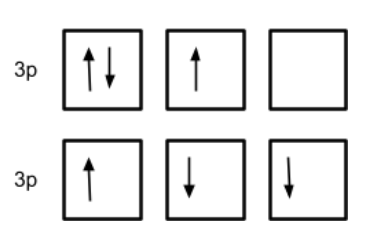
Top: Pairing up without at least 1 in each available orbital
Bottom: Electrons must all be spinning the same direction
What is the noble gas notation for selenium?
[Ar] 4s2 3d10 4p4
The following is an example of breaking which rule?
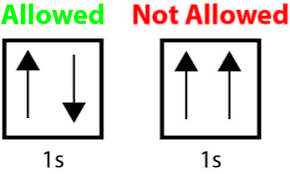
The Pauli Exclusion Principle
What is the order - from lowest to highest energy - of the following 3 orbitals?
4f 5d 6s
6s, 4f, 5d
What is the electron configuration for Osmium?
1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 6s2 4f14 5d6
What would an f orbital with 11 e- in it look like?
4 pairs of e-, 3 lone e-
What is the noble gas notation for gold?
[Xe] 6s2 4f14 5d9
The following picture shows a violation of which rule? Explain WHY.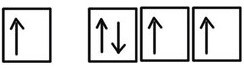
The Aufbau Rule; The p-orbital should not be filled until the s-orbital is filled