What four letters represent the orbitals?
s,p,d,f
What rule is being broken?
Each orbital needs an electron before pairing electrons
The arrows in orbital diagrams are replaced with _______ in electron configurations.
exponents/ superscripts
What group in the periodic table are the noble gases found in?
Group 18/8A

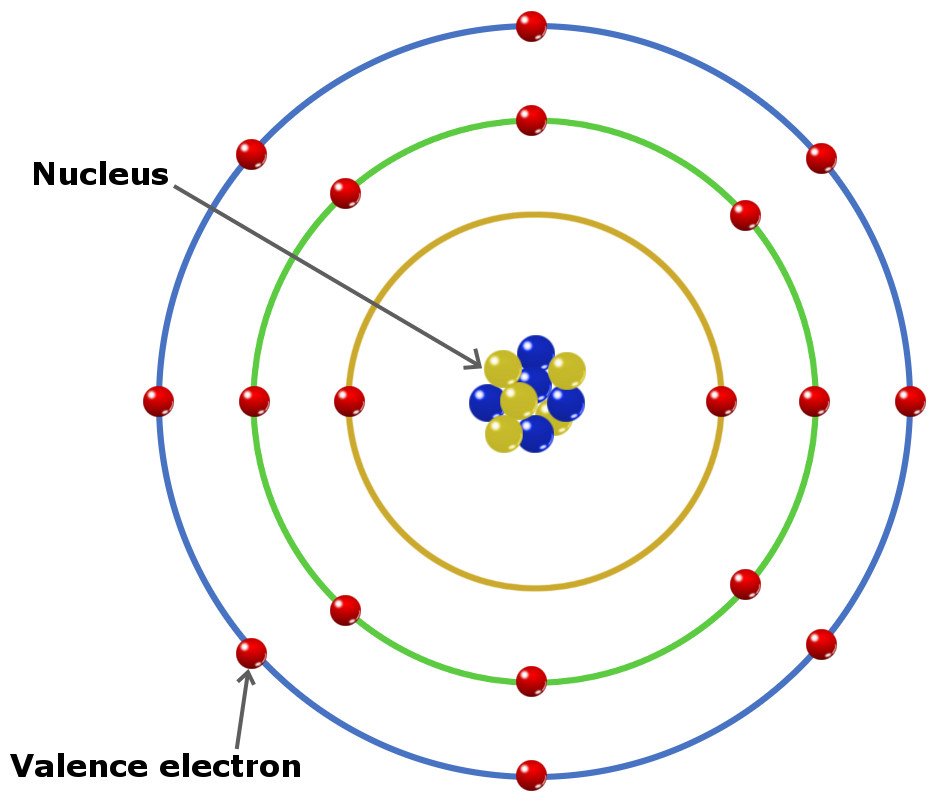
Valence electrons are found where?
Outermost energy level
What orbital is being shown?
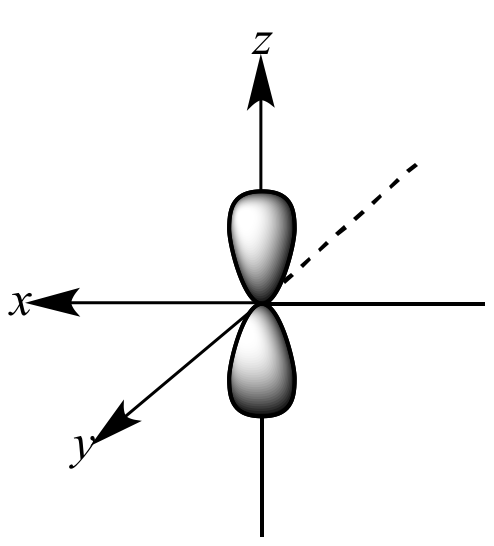
p orbital
Why is the top OFD incorrect?

2s orbital has two +1/2 spin electrons when it needs one +1/2 and one -1/2
Write the electron configuration for Boron (B)
1s22s22p1
What noble gas would be used to write the noble gas configuration of Arsenic (As)?
Argon (Ar)
Use the noble gas notation to determine the number of valence electrons.
[He]2s2
2
6
What element has the following orbital notation?
Carbon
What element has the following unabbreviated electron configuration?

Phosphorus
What element has the following noble gas configuration?

Antimony (Sb)
Use the noble gas notation to determine the number of valence electrons.
[Kr]5s24d105p5
7
How many total electrons can all d orbitals hold?
10
Draw the orbital filling diagram for Sulfur.
Sulfur
Write the full electron configuration for Nitrogen.

What element has the following noble gas configuration?
Chlorine (Cl)
Draw the electron dot diagram for Neon with a noble gas configuration of
[He]2s22p6

How many total electrons can all f orbitals hold?
14
Draw the orbital filling diagram for Iron
 Iron
Iron
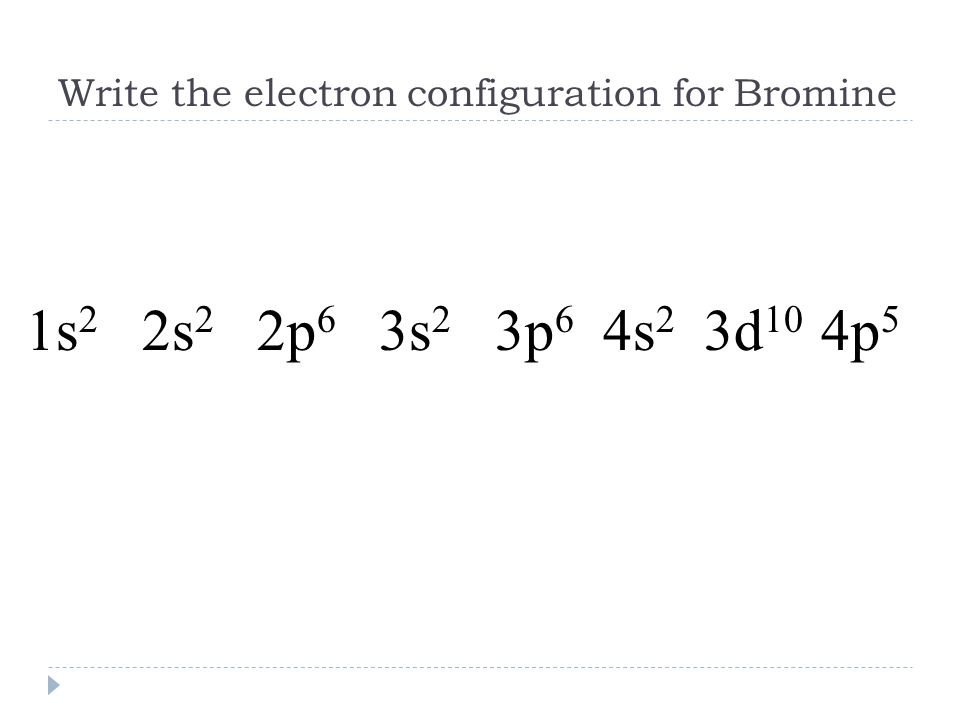
Write the full electron configuration for Bromine (Br).
Write the noble gas notation for Aluminum.

Draw the electron dot diagram of Vanadium (V) with the electron configuration of
[Ar]4s23d6
2 valence electrons (paired)