Two attributes of an electromagnetic wave.
With Team Gnilleps 100
What are wavelength and frequency?
The condensed electronic structure and the number of valence electrons in an atom of sulfur?
With Mindmeld 100
What is [Ne]3s23p4
The nuclide notation for magnesium-26.
With guess that song bonus of 100.
What is 2612 Mg?
1s2 2s2 2p6 3s2 3p3
What is the full electronic structure of phosphorus?
Na2CO3
With Guess That Song 100
What is sodium carbonate.
The wavelength in meters of a 95.1 MHz EM wave.
What is 3.5 m?
Distinguish between a continuous spectrum and a line spectrum.
What is radiation spread over all wavelengths/frequencies/energies/colours
Line spectrum: is radiation (absorbed/emitted) at specific wavelengths/frequencies/energies/colours
Rule that explains that only one electron occupies degenerate orbitals before pairing
What is Hund's rule or the Mulan Rule?
The condensed electron configuration of Te2- ion.
With guess that song bonus 300 points
What is [Kr] 5s2 4d10 5p6?
Magnesium and nitrogen combined to make a product. Given the chemical formula and the name of the product.
What is Mg3N2 and what is Magnesium nitride?
IR, VISIBLE, UV, GAMMA, RADIO in order of increasing frequency.
With guess that song bonus of 200.
What is Radio < IR < VIS < UV < Gamma
The discrete bands of light formed when an element's electrons in an atom move from their excited state to another energy level.
What is line spectra (or atomic emission spectra)?
Fill in the table of the mass and charge of the atomic particles.
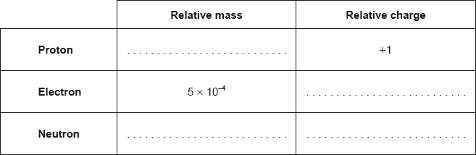
What is 1, -1, 1 and 0?
Full electronic configuration and number of unpaired electrons in Cr3+
What is 1s22s22p63s23p63d3 and three electrons?
SF5
What is sulfur pentaflouride?
The wavelength of light in nm having a frequency of 3.5 X E14 Hz.
With team Gnilleps of 200
What is 860 nm?
The name given to the n = infinity energy level in a gaseous atom.
With guess that song bonus of 200.
What is ionization energy?
Identify the invalid orbitals from the following list:
1s 3p 1p 4d 3f 4d
With Guess that Song 200
What are 1p and 3f?
The maximum number of electrons that can be held in the n = 3 shell.
With Guess that Song 300
What is 18 electrons?
SO42-
With GNILLEPS 300
What is the sulfate anion.
The wavelength in meters of heated lithium atoms that emit photons of light with an energy of 2.96 x 10-19 J.
What is 6.72 x 10-7 m?
The total number of electrons in p orbitals in an atom of iodine?
What is 23?
Mass spectroscopic analysis of a sample of magnesium gave the following results:
Mg-24 78.60
Mg-25 10.11
Mg-26 11.29
What is the atomic mass of Mg?
What is 24.33?
What is the atomic number of the element of transition metal ion X2+ that has the electronic configuration [Ar]3d9?
What is 29?
Name the compound and give the names of the cation and anion that make it up including the charge, NH4OH.
What is ammonium hydroxide?
NH4+ and OH- (ammonium and hydroxide or hydroxyl).