The electron configuration for F
what is 1s2 2s2 2p5?
The element for [Ne] 3s2 3p4
What is sulfur?
The Lewis Model for potassium.
What is K with 1 valence electron?
What is valence electron from left to right of periodic table -group 1, group 2, group 3, group 4, group 5, group 6, group 7, group 8
1, 2, 3, 4, 5, 6, 7, 8
Number of rings on the Bohr Model for silicon.
What is 3?
The element that has a configuration of 1s2 2s2 2p6 3s2 3p6
what is Ar?
Noble gas configuration for Cobalt.
[Ar] 4s2 3d7
How/when should you pair electrons?
Place your first 4 electrons SOLO and then pair them for 5-8
What group has 4 valence electrons? Name two elements in this group.
Group 4/14. Carbon, Silicon, Germanium, Tin, Lead, Flerovium
Number of protons, neutrons, electrons in the nucleus for tin.
What is protons= 50? What is neutrons= 69? What is electrons= 50?
What is Nickel
The noble gas configuration of Sn
True or false.
The Lewis dot diagram for I has 1 valence electron.
False it has 7
Describe how to use the periodic table to find the energy and valence electrons of an element.
The row/period equals the energy level/number of rings on the Bohr model.
The group number indicates the number of valence electrons/dots around the Lewis dot diagram?
Draw the Bohr model for Be.
Be
The electron configuration of Sn
What is 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p2
Noble Gas configuration of Po.
What is Po- [Xe]6s24f145d106p4
Draw a Lewis Dot for Arsenic
As with 5 valence electrons
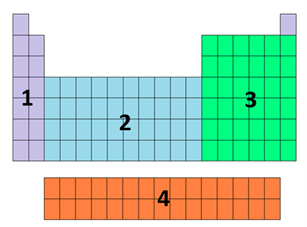
the block represented by each number
1 = s
2 = d
3 = p
4 = f
Draw the Bohr model for Cl.
Cl
Electron Configuration for Ba
1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 6s2
Noble gas configuration for Fe
[Ar] 4s2 3d6
Lewis model for bromine.
What is Br with 7 valence electrons?
What group has 8 valence electrons? What do we call these elements?
Group 8/18. The Noble Gases.
Draw the Bohr model for Calcium
4 rings, 2 valence