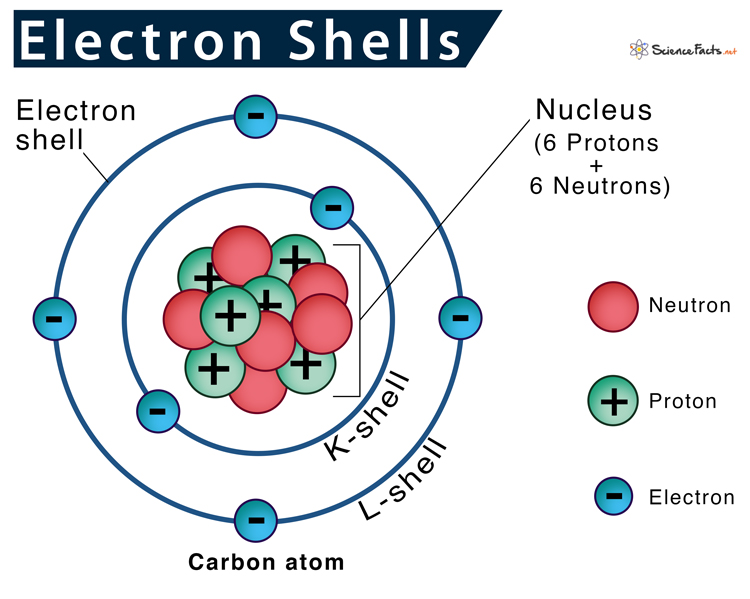
 found in the outermost ring of an atom
found in the outermost ring of an atom
What is a valence electron?
 a positively or negatively charged particle
a positively or negatively charged particle
What are ions?
This property allows ionic compounds to conduct electricity when dissolved in water.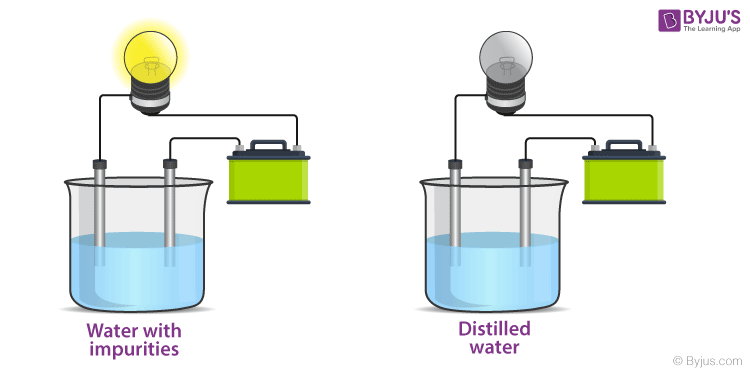
What is electrolytic conductivity?
 has 2 valence electrons.
has 2 valence electrons.
What is group 2?
 Ionic compounds tend to have this type of melting and boiling points
Ionic compounds tend to have this type of melting and boiling points
Ionic compounds tend to have this type of melting and boiling point?
What is the formal name of group 1 elements
What are alkali metals?
Potassium Chloride
What is an example of ionic bonding?
Ionic compounds form this type of repeating structure due to the strong attraction between oppositely charged ions.
What is a crystal lattice?
how many valence electrons are on chlorine
What is 7 electrons
when a metal or nonmetal combine by ionic bonding and the result has different properties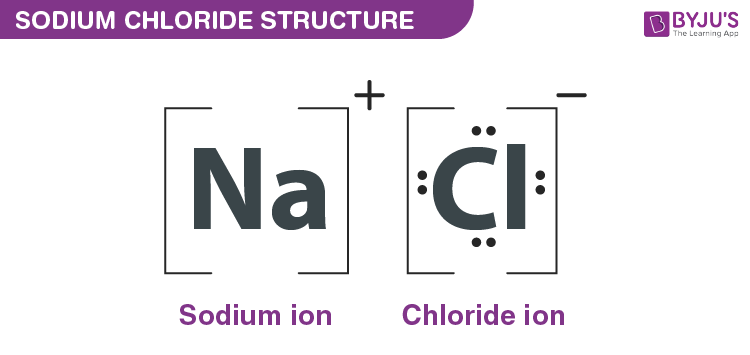
What is forming an ionic compound?
Positively charged ions![]()
What is cations?
 Many ionic compounds dissolve in this type of solvent due to its ability to surround and separate the ions.
Many ionic compounds dissolve in this type of solvent due to its ability to surround and separate the ions.
What is water (a polar solvent)?
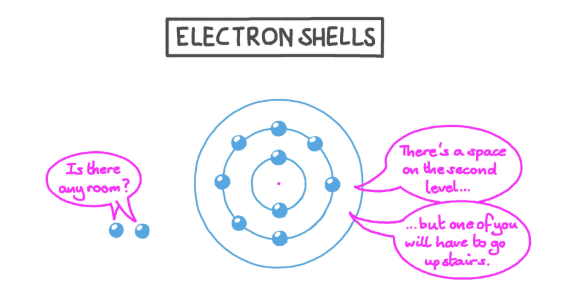 If you have 2 electrons in your outermost ring, are you full?
If you have 2 electrons in your outermost ring, are you full?
No, only for helium.
the elements in this group on the periodic table cannot form ions since they are already filled.
What is group 8?
 How much atoms of Mg and Cl are involved in the ionic bonding of Magnesium Chloride
How much atoms of Mg and Cl are involved in the ionic bonding of Magnesium Chloride
What is Mg 1 and Chlorine 2
Although solid ionic compounds do not conduct electricity, they can conduct electricity when in these two states.
What are molten (liquid) and aqueous (dissolved in water) states?