What is Ground State?
This is the name for electrons that are found at the outermost electron shell.
What are Valence Electrons?
What is groups; periods?
The electron configuration of Oxygen (O).
What is 1s22s22p4?
Physical property referring to a substance's ability to be bent and shaped.
What is Malleability?
An electron occupies the lowest-energy orbital that can receive it.
What is Aufbau's Principle?
This is the carrying capacity of the third innermost electron shell.
What is 18?
This is how many electrons Bismuth would have in its 6th electron shell.
What is 5?
The electron configuration of Magnesium (Mg).
What is 1s22s22p63s2?
A number used to determine the amount of subatomic particles in an atom's nucleus.
What is Mass Number?
The state electrons can be found when they absorb a photon, gain energy, and temporarily jump to a higher energy level.
What is Excited State?
This is how many types of orbitals can be found at the second electron shell.
What is 2?
(s, p)
A metalloid with 5 electron shells and 6 valence electrons.
What is Tellurium (Te)?
Electron configuration of Calcium (Ca) in Noble-Gas notation.
What is [Ar]4s2?
The nuclear force that overcomes the electromagnetic repulsion of like particles within 2 femtometers distance of each other.
What is Strong Force?
Orbitals of equal energy are all occupied by one electron before any of them are occupied by a second electron.
What is Hund's Rule?
This is the total number of electron shells that an atom can have up to.
What is 7?
A metal with 3 electrons in its 3rd shell.
What is Aluminum (Al)?
Electron configuration of Iron (Fe) in Noble Gas Notation.
What [Ar]3d64s2?
(OR [Ar]4s23d6 by way of chart)
An atom has 48 protons, 65 neutrons, and 48 electrons. This is its isotope name in hyphen-notation form.
What is Cadmium-113?
(Unstable with a very long half-life).

This is a particle like bundle of electromagnetic radiation.
What is Photon?
Electron shell(s) that can hold the highest amount orbital types.
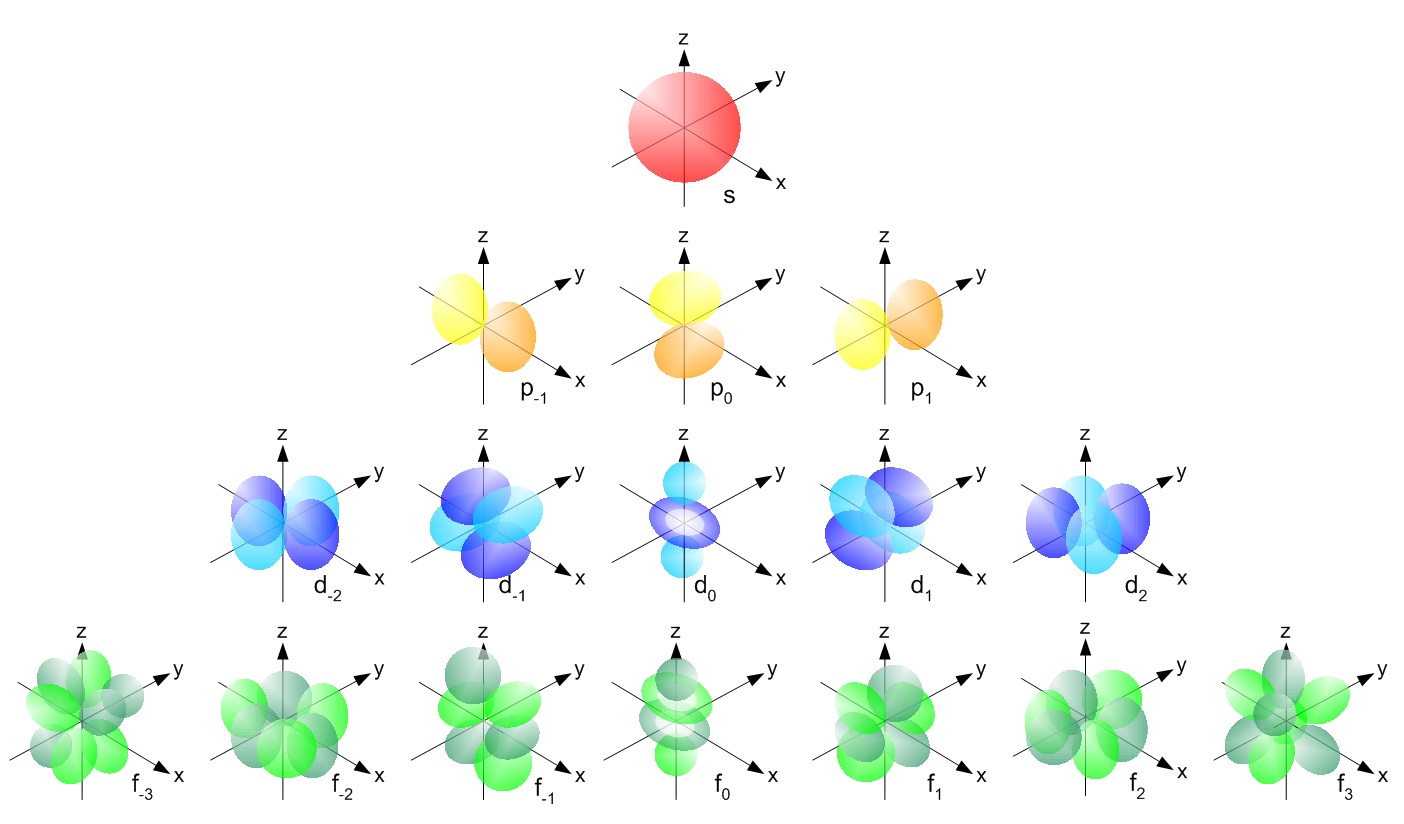
What is 4th and 5th shells?
A noble gas with 4 energy levels.
What is Krypton (Kr)?
Electron configuration of Tin (Sn) in Noble Gas Notation.
What is [Kr]4d105s25p2?
(OR [Kr]5s24d105p2 by way of chart)
Protium, Deuterium, and Tritium are different isotopes of this element.
What is Hydrogen?
Protium = H-1
Deuterium = H-2
Tritium = H-3
