Draw the Electron Dot Diagram for Ca.
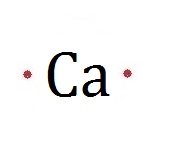
How many energy levels are in selenium (Se)?
4 energy levels
Metallic elements are generally (larger or smaller) than nonmetallic elements.
larger
5s2
strontium (Sr)
The s sublevel contains how many electrons?
2 electrons
Write the full electron configuration for bromine.
1s22s22p63s23p64s23d104p5
Metals ___ electrons while nonmetals ___ electrons to obtain a full valence shell.
lose, gain
Which of the following has the largest atomic radius: Mg, Ca, Sr, Ba
Ba
4p1
gallium (Ga)
_____ says that an electrons location and velocity cannot both be known.
Heisenberg's Uncertainty Principle
Which of the following orbital notations is correct?
e.
How is atomic radius measured?
the distance from the center of the nucleus to the outer boundary of the electron cloud
Which is larger: bromine ion or bromine atom?
bromine ion (Br-)
the element in period 4 and group 16
selenium (Se)
3 orbitals
Write the noble gas configuration for iron (Fe).
[Ar]4s23d6
Elements in the same group have the same number of ______.
valence electrons
Which has the largest ionization energy: Na, Mg, Al, Si
Si
5p5
iodine (I)
Which of the following has the highest energy: 6s, 5p, 4d, or 5d?
5d
Draw the orbital notation diagram for potassium (K).
The last electron of Uranium is entered into which sublevel?
the f sublevel
Which has the largest second ionization energy: Na, Be, Mg, P
Na (after losing one valence electron, it has a full shell and does not want to lose another)
the only metalloid in Group 13
boron (B)
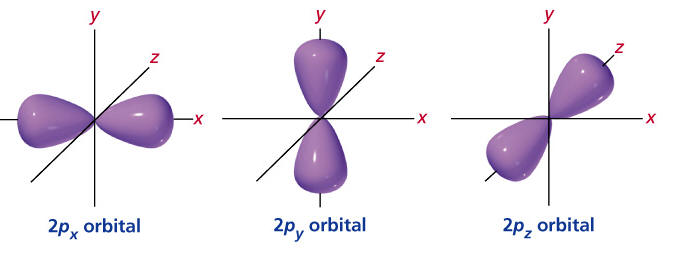
What shape is the cloud of the p sublevel?
peanut (or dumbell)