What element has the following electron configuration:
1s2 2s2 2p4
Oxygen
The s block has how many orbitals and electrons when filled?
1 orbital, 2 electrons
What is the name of group 1A in the periodic table?
Alkali Metals
What element is found in period 3, group 2?
Magnesium
Electronegativity and ionization energy increase ___ and ____
Up + Right
What is the electron configuration of Lithium?
1s2 2s1
The p block has how many orbitals and electrons when filled?
3 orbitals, 6 electrons
What is the name of group 8A in the periodic table?
Noble Gases
What element is found in period 5, group 14?
Tin
Which element has the largest radius:
Nitrogen or Oxygen?
Nitrogen
What is the noble gas electron configuration of Copper?
[Ar] 4s2 3d9
The d block has how many orbitals and electrons when filled?
5 orbitals, 10 electrons
The periodic table is organized by increasing______
Atomic number
What is the name group 7A on the periodic table?
Halogens
Which element has the largest ionization energy?
Sulfur, Chlorine, Bromine or Selenium
Chlorine
Identify the element:
[Ar]4s23d10
Zinc
What rule is being broken?
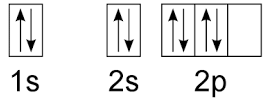
Hund's Rule
Atomic radius increases ____ and ______ in the periodic table?
Down + Left
What subatomic particle is responsible for the properties of an atom?
Electron
Which element has the smallest atomic radius?
Potassium, Calcium, Rubidium, Strontium
Calcium
What is the noble gas electron configuration of Silver (Ag)?
[Kr] 5s24d9
What rule is being broken in this diagram? Explain.
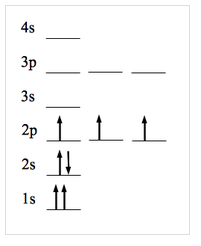
Pauli Exclusion Principle; 1s needs opposite spin
Why are noble gases special?
They have s and p orbitals filled. They are stable (nonreactive)
Mention 2 characteristics of metals
-good conductors of electricity and heat
-lustrous
Which element has the largest electronegativity?
Boron, Carbon, Silicon, Germanium
Carbon