The location of protons and neutrons in an atom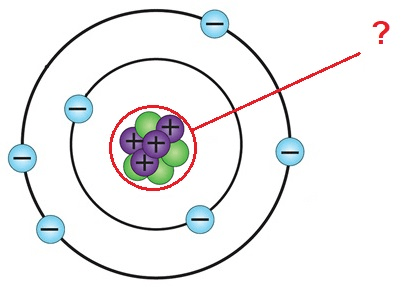
What is the nucleus?
The element in period 3 and group 3.
What is aluminum?
The letter or letters that represent an element
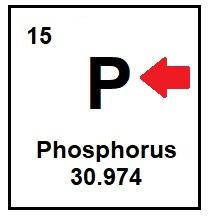
What is a symbol?
The trend for the atomic radius as it goes from left to right of a period and up vertically.
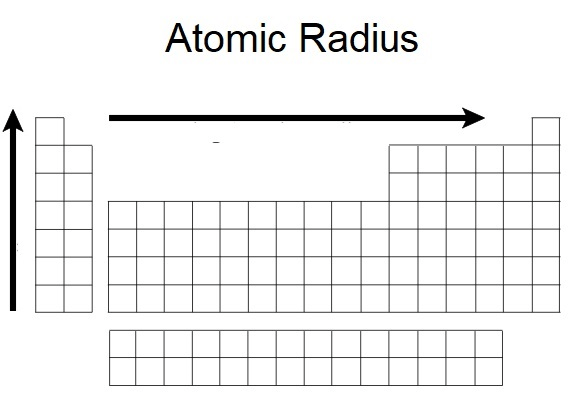
What is decreases?
The electrons in the outermost energy level of an atom that influence how an element will react with other substances
What are valence electrons?
The location of electrons in an atom
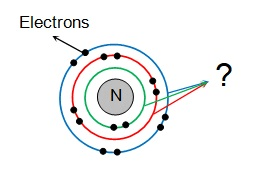
What is energy levels or shells?
The element that has 7 valence electrons and is in period 2
What is fluorine
The number of protons in an atom
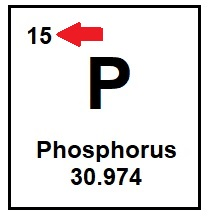
What is the atomic number?
The trend for the energy needed to remove an electron, ionization energy, as it goes from left to right and as it goes from bottom to the top
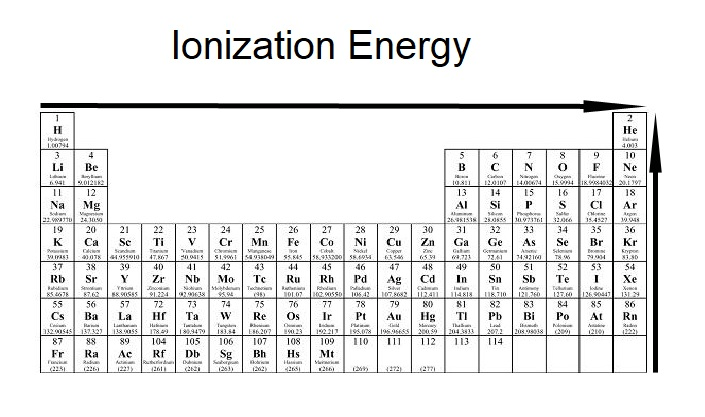
What is increases?
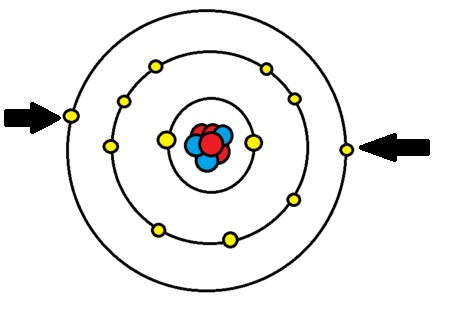
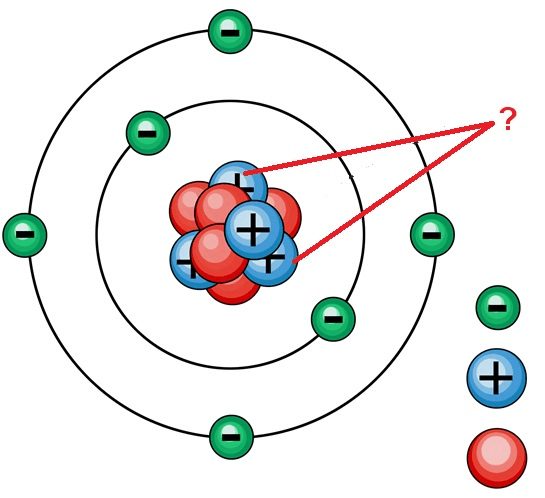
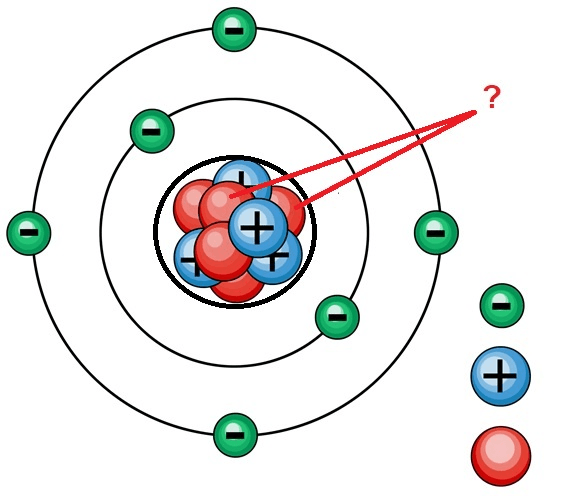
The element represented by the Bohr's model below
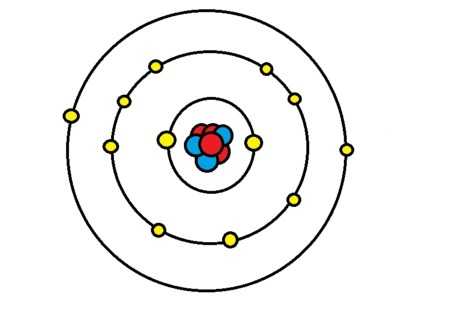
What is Magnesium?
The positively charged particle in an atom.
What is a proton?
The neutral element that has 20 electrons
What is Calcium?
The term used for the rows of the periodic table
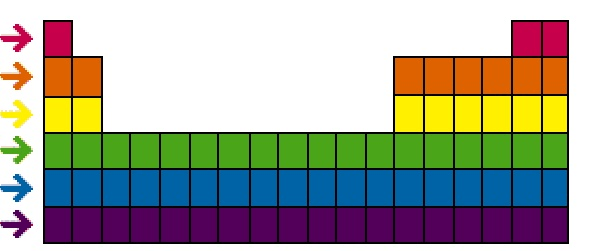
What are periods?
The electronegativity trend as it goes from right to left and as it goes from top to bottom
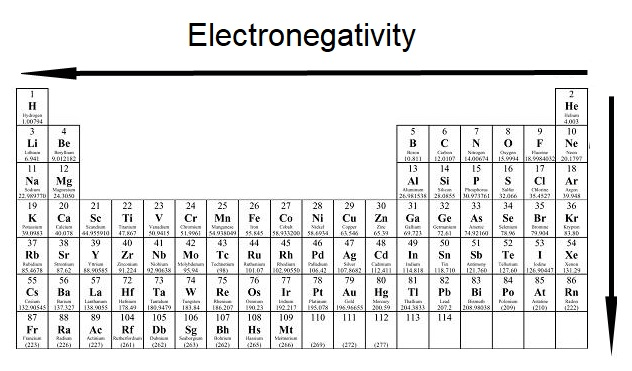
What is decreases?
The element that would have the Lewis dot structure configuration of X
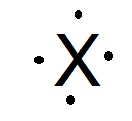

What is Silicon?
The subatomic particles that do not have positive or negative charges, they are neutral.
What are neutrons?
The element that is in period 2 and will lose 2 valence electrons to have a full energy level
What is Beryllium?
The name for the columns on the periodic table
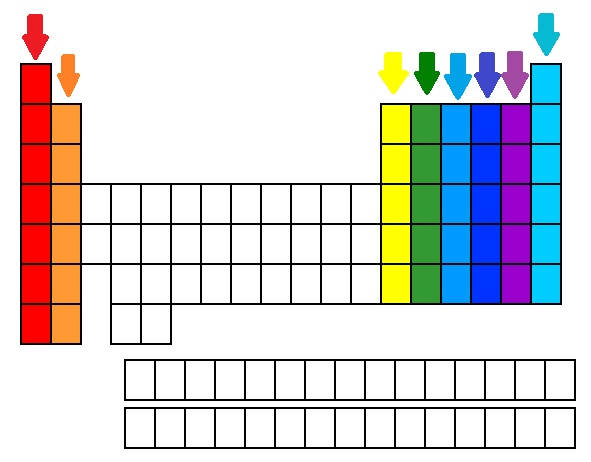
What are groups?
Base on the trend, which element would have the lowest electronegative value or tendency to attract electrons.

What is Li or Lithium?
The reason why Francium is the largest atom in group 1
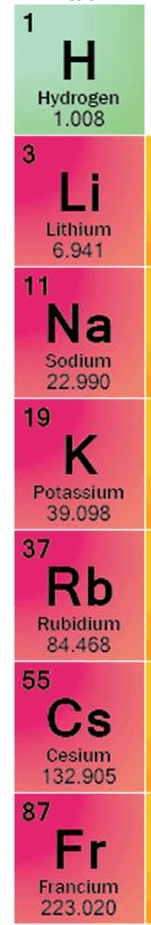
What is it has more energy levels or shells?
The negatively charged particles found in an atom.
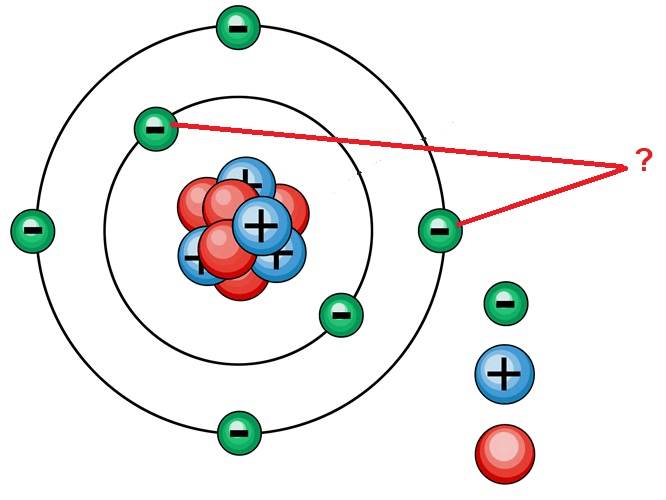
What is an electron?
The element in period 2 that needs to gain 2 valence electrons to have a full outer energy level
What is Oxygen?
The average mass of an element based on the abundance of each isotope
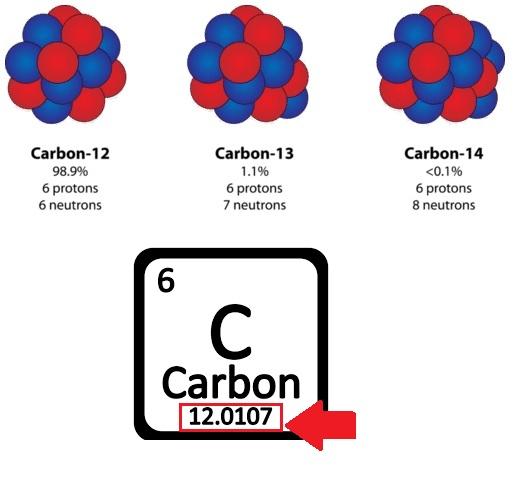
What is the atomic mass?
Based on the elements below, the element that has the highest ionization energy.

What is Argon?
The similarities between Element 1 and Element 4 or the similarities between Element 2 and Element 3.
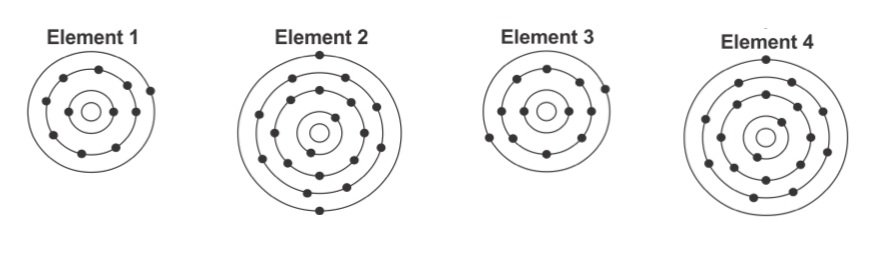
What is the number of valence electrons?