These are defined as one of the "pure" substances in nature... they cannot be broken down into simpler substances.
What are elements?
The atomic mass and the atomic number.
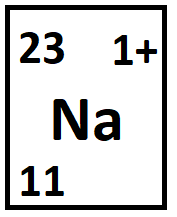
What are 23 and 11?
A = 23
Z = 11
The name given to an ion with more electrons than protons.
What is an anion?
A device used to measure mass in our chemistry class.
What is an electronic balance?
Chlorine has a relative atomic mass of 35.4
Which of the following chlorine isotopes is more common? 35Cl or 36Cl
What is 35Cl?
The smallest piece of an element that still retains the properties of that element.
What is an atom?
The maximum number of electrons found in the first energy level.
What is 2?
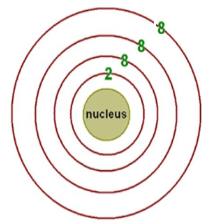
The number of protons and the number of neutrons.
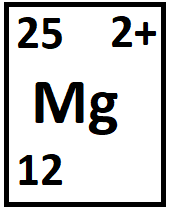
What are 12 and 13?
p = 12
n = 25 - 12 = 13
The length of this line:
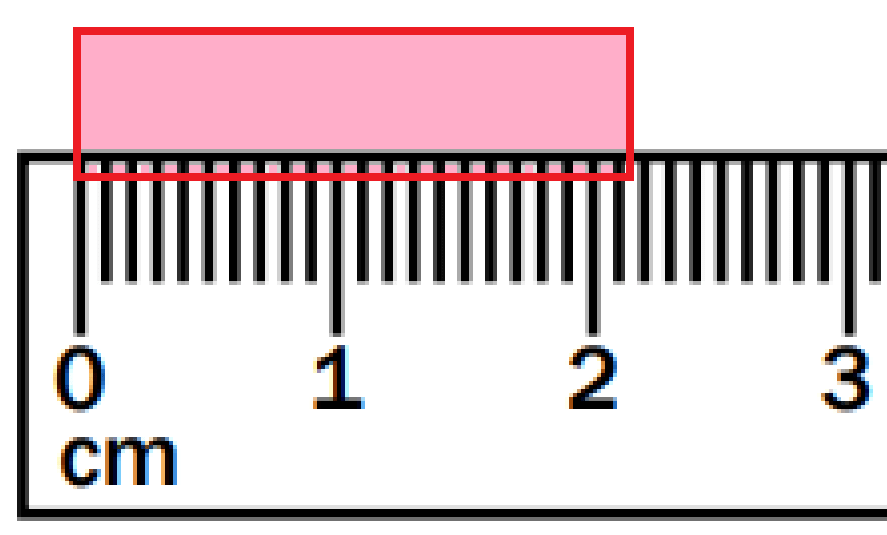
What is 2.15 cm?
These two isotopes have the same number of this subatomic particle.
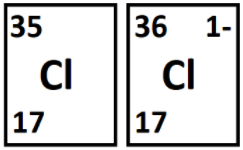
What are protons?
The chemical symbols of sodium, calcium and potassium.
What are Na, Ca and K?
Atoms with the same atomic number but different atomic masses.
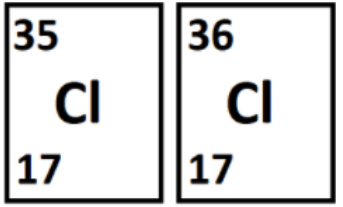
What are isotopes?
The number of protons and the number of electrons.
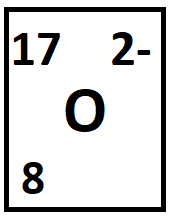
What are 8 and 10?
p = 8
e- = 8 + two extra electrons = 10
The fourth stage of a mass spectrometer.
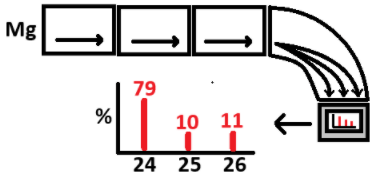
What is deflection?
The mass of a water molecule.
H2O
What is 18?
H2O = 1 = 1 + 16 = 18
The atomic mass and charge of the ion.
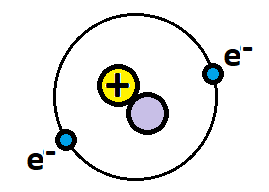
What is 2 and 1-?
A = 2
There is one extra electron, so the charge is 1-.
The names of the elements used to spell this word:

What are Calcium and Boron?
Jeopardy!
Only your team may answer this question, and you get to choose how much you bet.
But be careful, if you get it wrong then you will lose that many points!
The number of electrons found at X in the following 1+ cation.
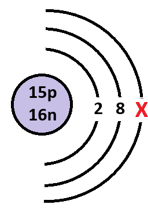
What is 4?
The technique of placing an object in water to find its volume.
What is water displacement?
the object displaces, or pushes away, the same amount of water as its volume
The four main gases found in the atmosphere.
(not including water vapor)
What are nitrogen, oxygen, argon and carbon dioxide?
N2 78% O2 21% Ar 0.70%
CO2 0.03%
The atomic number of a 2- ion with electrons 2, 8, 8.
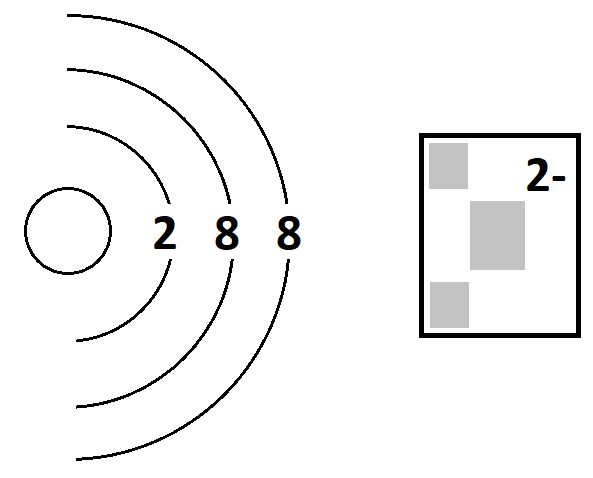
What is 16?
A = 16
The names of the elements used to spell this word:

What are Sodium, Phosphorus, Potassium, Iodine, Nitrogen?
Na P K I N
(Na P K In... Indium)
The atomic mass and atomic number of this atom:
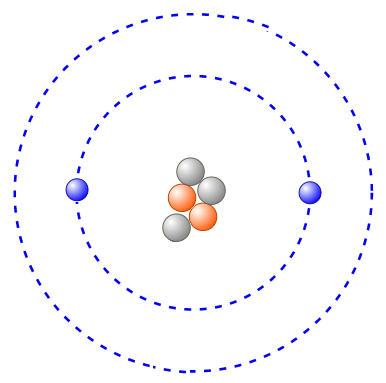
What are 5 and 2?
The atomic mass (the number of protons plus the number of neutrons) is 5.
The atomic number is 2. There are two protons in the nucleus. We also know this is an atom, so the number of protons must equal the number of electrons.
Jeopardy!
Only your team may answer this question, and you get to choose how much you bet.
But be careful, if you get it wrong then you will lose that many points!
The mass of 70.0 ml of water.
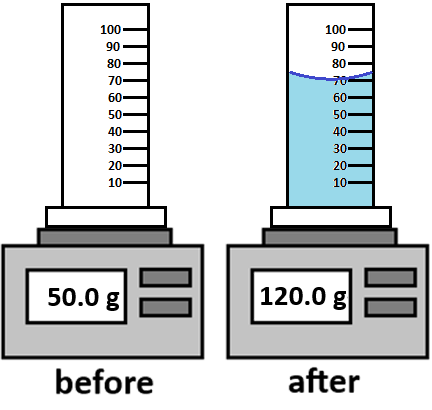
What is 70.0 grams?
The density of water is 1.0 g/ml.
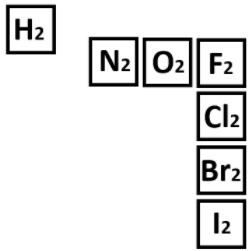
The diatomic elements.
What are H2 N2 O2 F2 Cl2 Br2 I2?
The number of neutrons in the following ion.
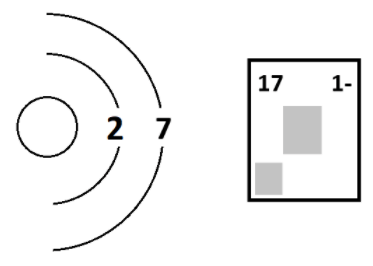
What is 9?