Shiny, malleable, ductile, conductive of both heat and electricity
Example: Iron, copper, and Sodium
What is a Metal
A pure substance composed of all the same kind of atom
What is an element?
Vertical columns on the periodic table are called?
What are families?
3H2SO4 How many molecules?
3
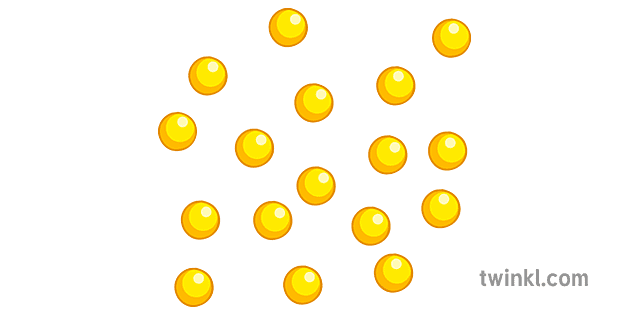
Element
Symbol for Lead
Pb
Dull, brittle, not a conductor of heat or electricity.
Example: Carbon, Sulfur, and Hydrogen
What is a Nonmetal
Two or more elements chemically bonded together that cannot be separated by physical means
What is a compound
Members of a group on the periodic table have this in common.
Similar physical properties, Same number of valence electrons, similar ways of bonding to form compounds.
3H2SO4 How many Sulfur atoms?
3
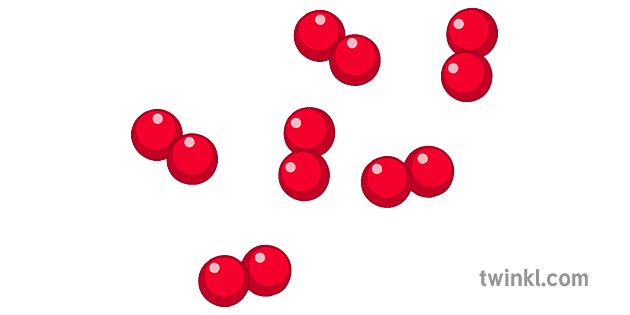
Element (diatomic)
Symbol: Fr
Francium
Displays some characteristics of both metals and nonmetals, called Semiconductors
Example: Boron, Silicon, Germanium
What is a metalloid?
Any combination of elements or compounds or both that exist together without being chemically bonded
What is a mixture?
Group 18
Noble Gasses
Ca(C2H3O2)2 How many Oxygen atoms?
4
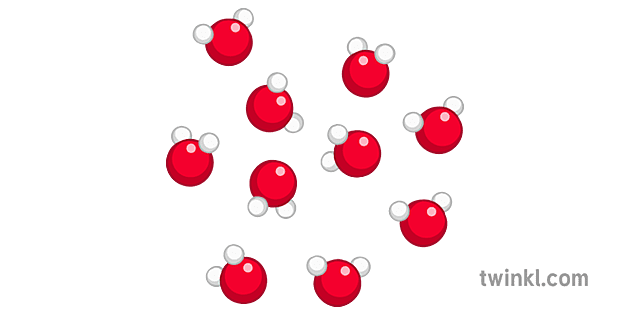
Compound
Element found in period 5 with 3 valence electrons
Indium
A letter or letters used in a compound formula to indicate which elements are present in the compound
What is an elemental symbol?
Two atoms of the same kind that occur bonded together in nature
What is a diatomic elemental molecule?
Group 1
Alkali metals
5Ga2(Cr2O7)3 How many Chromium atoms?
30

Mixture of elements
alkali metal in period 2
Lithium
A number to the lower right of an elemental symbol that tells you how many atoms of that element are in a compound
What is a Subscript?
The substance that is dissolved when a solution is formed
What is a Solute?
Group 2
Alkaline Earth Metals
4Fe2O3 Total number of atoms?
20
Mixture of elements
group 8; period 4
Iron
Number to the left of a compound formula that tells you how many molecules you have of that compound.
What is a coefficient?
In a solution, the substance in which another substance is dissolved
What is a solvent?
Elements found around the red line on the periodic table.
Metalloids
7C2S2 What does C and S represent?
Elements, Carbon/Sulfur
Mixture of elements and compounds
Symbol for Titanium
Ti
Drawings or illustrations used to represent ideas or concepts
What is a visual model?
A mixture that appears uniform (same) throughout, a solution
What is homogeneous?
Most reactive metal
Francium
3NaHCO3 How many elements are present?
4
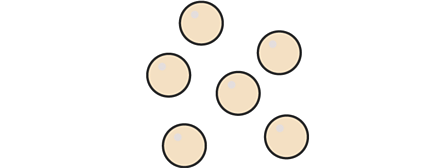
Element
U
Uranium
Tangible objects used to represent a scientific phenomenon, such as nuts, bolts, and washers to represent atoms
What is a physical model?
Describes a mixture in which the component parts are visible
What is heterogeneous?
Most reactive Nonmetal
Fluorine
5Al2(SiO3)2 Total number of atoms?
50
Mixture of compounds
Ar
Argon

