Which substance is a mixture?
sugar
salt
water
milk
What is millk
which of these is a compound?
C
B
Fe
CO2
What is CO2
which substance is classified as an element?
NaCl/ CO/ H2O/ Be
What is Be
What type of change is baking a cake?
chemical change
which combination of substances is a compound?
A. salt and water stirred in a glass
B. food coloring in frosting
C. peanut butter and jelly sandwich
D. sulfur dioxide and water forming acid rain
What is D. sulfur dioxide and water forming acid rain
which of these represents a chemical change?
A. a match is lit, creating a yellow-orange flame
B. a small piece of ice melts, changing from a solid to a liquid
C. a ball of clay sinks in water, and floats when reshaped.
D. a pot of water is heated to boiling, and evaporation occurs
What is A. a match is lit, creating a yellow-orange flame
which option contains only compounds?
A. H2O, H30, CO2
B. Ni, Li2, NaCl
C. NH3, He, C6H12O6
D. Ne, H, He, C
What is A. H2O, H30, CO2
which of these is a compound and not a mixture?
A. chocolate milk
B. table salt
C. vinegar with oil
D. sand in water
What is B. table salt
which statement best describes a physical change?
A. a process separates water into hydrogen gas and oxygen gas
B. an iron nail exposed to the atmosphere forms rust.
C. burning carbon releases the gas carbon dioxide.
D. salt added to a cup of water makes salt water.
What is D. salt added to a cup of water makes salt water.
What type of change is making icing?
physical change
the chemical process of burning coal produces energy and ash. the ash produced is best described as a...
A. new state of matter
B. new type of element
C. substance with properties identical to coal
D. substance with properties different from coal.
What is D. substance with properties different from coal.
a student stirs together salt crystals and sugar crystals. the crystals are the same size and color. together the salt and sugar make a ...
solution/ compound/ mixture/ molecule
What is mixture
which process most likely produces a physical change, without a chemical change?
A. a solid is exposed to heat and transforms into a liquid
B. two liquids are mixed together and a solid forms in the solution
C. a metal burns in air and produces a white powder
D. a metal is exposed to water and becomes corroded
What is A. a solid is exposed to heat and transforms into a liquid
a student observes that a gas if formed when chemical Y is added to chemical Z in the lab. The student's observation of a new product being formed is the common outcome of all __________ changes.
physical/ chemical/ mass/ phase
What is chemical
which statement best describes the differences between a mixture and a compound?
A. compounds are pure element
B. compounds contain elements bonded together
C. compounds can be easily separated into distinct parts
D. compounds have multiple boiling points
What is B. compounds contain elements bonded together
Name the two types of pure substances.
What are elements & compounds?
a combination of salt and sugar grains is best classified as a mixture because...
A. the grains taste different from one another
B. both the salt and sugar grains are in the same state of matter
C. no new chemical bonds are formed between the salt and sugar grains
D. a new product is formed when the salt and sugar grains are combined.
What is C. no new chemical bonds are formed between the salt and sugar grains
a student applies heat to a solid substance. which indicates that a chemical change occurred?
A. the substance becomes a liquid but solidifies when cooled
B. the substance changes color and stays that color when cooled
C. the substance goes from liquid to gas very quickly
D. the substance expands but stays the same color
What is B. the substance changes color and stays that color when cooled
students heated a white solid. the solid produced a flame during the heating process. the product cooled into a black solid. which statement best classifies this change?
A. the change was only physical because the heat produced a color change.
B. the change was only physical because the properties of the substance were permanently changed
C. the change was only chemical because the substance remained in its original state
D. the change was only chemical because a flame was produced
What is D. the change was only chemical because a flame was produced
a student performed a classroom investigation by mixing a purple liquid and a white powder. the result was a blue solid. the student could best classify this reaction as a _________ change.
physical/ weight/ mass/ chemical
What is chemical
a student is classifying the two substances, H2O and O2, based on their chemical formulas. which correctly classified these substances?
A. both H2O and O2 are elements
B. both H2O and O2 are compounds
C. H2O is a compound and O2 is an element
D. H2O is an element and O2 is a compound
What is C. H2O is a compound and O2 is an element
What type of mixture is made up of different parts?
heterogenous mixture
two students work on different substances in a science lab. student 1 has a mixture and student 2 has a compound. both students will separate the parts of their substances. which best describes a result of the separation processes?
A. only the mixture will go through a chemical change
B. only the mixture will release heat when the parts are separated
C. each part of the compound will gain mass during separation
D. each part of the compound will have different chemical properties
What is D. each part of the compound will have different chemical properties
hydrogen and water combine to form water. which statement best explains how this reaction is similar to all chemical reactions?
A. matter is created.
B. energy is released
C. the product is in a different state than the reactants
D. the product has different properties from the reactants
What is D. the product has different properties from the reactants
a science teacher demonstrated physical and chemical changes by using a fork to hold a marshmallow over alit candle. which observation is the best evidence of a chemical change?
A. some candle wax melted
B. metal in the fork became warmer
C. the marshmallow became sticky on the inside
D. the marshmallow developed a brown crust on the outside
What is D. the marshmallow developed a brown crust on the outside
a strip of magnesium burns at a temperature of about 2220C. burning magnesium produces a brilliant white light and leaves behind a white powder known as magnesium oxide. which best explains why the burning of magnesium results in a chemical reaction?
A. a new substance is formed
B. a temperature increase occurred
C. the appearance of the magnesium strip changed.
D. the state of matter of the magnesium strip changed
What is A. a new substance is formed
a student observes actions that occurred in four different eggs. which action describes only a chemical change?
A. cut up egg
B. stirred egg
C. rotting egg
D. dropped egg
What is C. rotting egg
Brass is an alloy that is made up of two metals, copper and zinc. It is a mixture because it is uniform throughout. What type of mixture is brass?
homogenous mixture
Danny has acid reflux. What would be best to neutralize his issue?
Acid
Base
Water
Base
What is the pH scale?
A scale that measures whether something is an acid, a base, or neutral. Scale from 0-14.
I have an acid. What numbers could it fall on on the pH scale?
Any number below 7
Sublimation is a phase change where a solid, like dry ice, changes to a gas at room temperature. What type of change is sublimation?
physical change
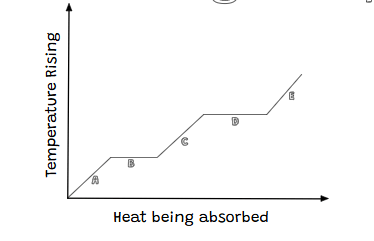 Tell me about letter B in the graph.
Tell me about letter B in the graph.
Letter B is a phase change and it is a solid melting into a liquid with no temperature change.
What is the Law of Conservation of Mass?
Mass is neither created nor destroyed. How much you start with is equal to how much you end with.