The reason we use fire for the flame test lab.
The kind of EM radiation we can see.
Visible
The electron configuration for Oxygen
1s22s22p4
The number of electrons in the highest energy level
Valence electrons
The rule violated in this image: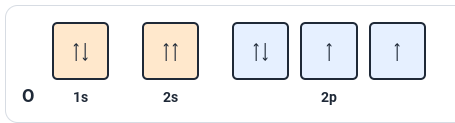
Pauli exclusion principle
This is the relationship between frequency and wavelength.
Inverse realtionship
This kind of EM radiation causes sunburns.
Ultraviolet (UV)
The electron configuration for Sulfur
1s22s22p63s23p4
The rule that says we have to fill the lowest energy level first.
Aufbau principle
What rule is violated in this image?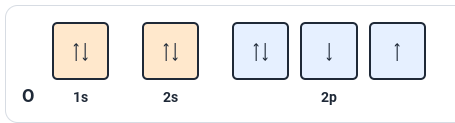
Hund's rule
The form of energy given off by electrons when they return to their ground state.
photons of light
This form of electromagnetic radiation has the lowest energy
Radio
The noble gas configuration for Sodium
[Ne]3s1
The rule that says no two electrons can have the same quantum numbers.
Pauli exclusion principle
What rule is violated in this image?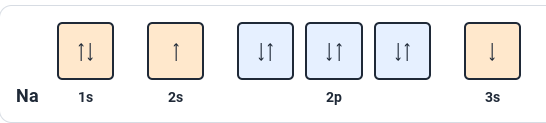
Aufbau Principle
The relationship between frequency and energy.
Direct relationship
This EM radiation has the most energy
Gamma
The noble gas configuration for Krypton
[Ar]4s23d104p6
The rule that says each orbital must first be filled with an electron with a parallel spin before they can be paired.
Hund's rule
What's wrong with this orbital diagram?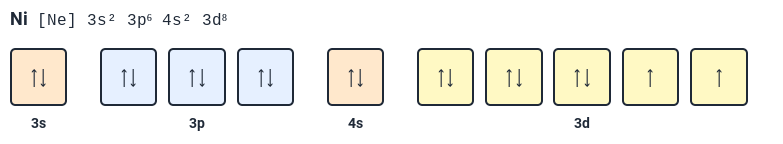
Wrong noble gas configuration
The speed of light.
What is 3.0x108 or 300,000,000 m/s
This kind of EM radiation is produced by humans but is invisible to our eyes.
Infrared
The electron configuration for Zinc.
1s22s22p63s23p64s23d10
The name of the shortcut in electron configuration
Noble Gas Configuration
What rule is violated in the orbital diagram in this image?
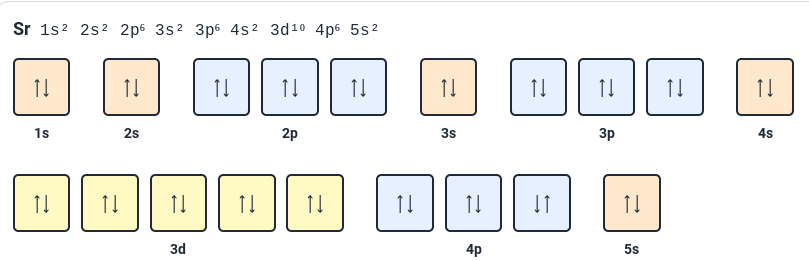
Nothing.