The atomic symbol for this element is Mn.
What is manganese?
A solution with a [H3O+] concentration of 3.58x10-3.
What is acidic?
The chemical formula for this polyatomic ion is "OH-"
What is hydroxide?
This type of reaction produces CO2 and water.
What is a combustion reaction?
This state of matter has vibrational energy but not rotational nor translational energy.
What is a solid?
These elements are diatomic.
What are N2, O2, F2, Cl2, Br2, I2, and H2?
(BrINClHOF)
H2SO4 is an example of this.
What is an acid?
The chemical formula for this polyatomic ion is SO42-.
What is sulfate?
The products of a neutralization reaction.
What are water and salt?
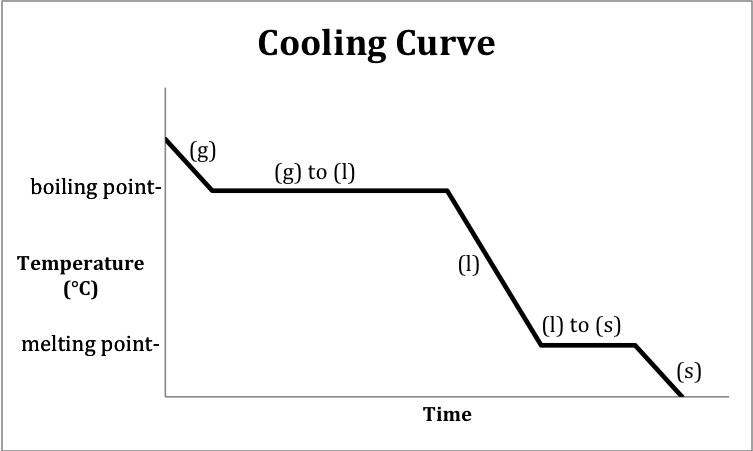 The phase change that occurs at the boiling point on this cooling curve.
The phase change that occurs at the boiling point on this cooling curve.
What is condensation?
This is the most electronegative element on the periodic table.
What is fluorine, F?
HCO31- is an example of this.
What is amphoteric/amphiprotic?
Transition metals are known to have variable charges. However, this particular transition metal always has a 2+ charge.
What is zinc, Zn?
A reaction whose products are at higher energy than the reactants.
What is endothermic?
When a gas is compressed at constant temperature, this will occur.
What is an increase in pressure?
The name of the elements in group 18.
What are noble gases?
This common molecular compound has an equal concentration of hydronium and hydroxide ions.
What is water?
A type of chemical bonding that involves the electrostatic attraction between oppositely charged ions or between two atoms with very different electronegativities.
What is ionic bonding?
Consider the following system at equilibrium:
Fe3+(aq) + SCN-(aq) → FeSCN2+(aq) + heat
Increasing the concentration of this will shift the reaction to the left.
What is FeSCN2+?
Or...
What is heat?
All elements are composed of these three things.
What are electrons, neutrons, and protons?
The atomic symbol for this element is Yb.
What is Ytterbium
This acid is responsible for the carbonation in fizzy soda drinks.
What is carbonic acid?
Fe3(PO4)2 is composed of these ions.
What is Fe2+ and PO43- ?
This type of reaction produces an insoluble precipitate.
What is a double-replacement/double-displacement reaction?
The temperature at which all heat and motion is absent.
What is absolute zero? (O K)