Endothermic
Exothermic
Phase changes
Bonus
100
heat is __
absorbed
100
product are getting __
hotter
100
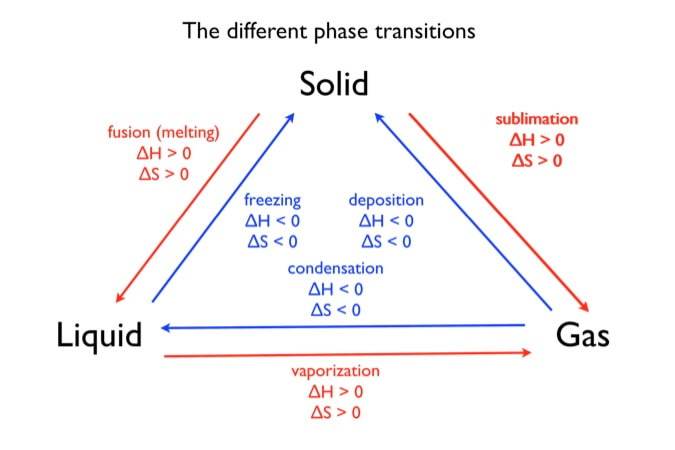
What are the 3 phase changes
Solid, liquid and Gas
200
Define Activation Energy
The energy needed to make reaction happen
200
Where does the product go in an exothermic equation?
At the end, heat is the product.
200
Endothermic bonds are __
Breaking
300
Draw an endothermic graph

300
Draw an exothermic graph
300
Going from a solid ---> liquid what is happening?
Melting
400
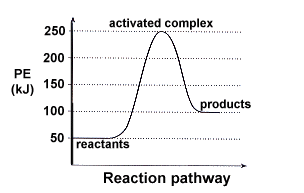
What is the activation energy value for the forward reaction?
250-50=200 KJ
400
What is the activation energy value and the change in heat value for the forward reaction?
500-400=100KJ (activation energy)
100-400= -300 KJ( change in heat)
400
Going from a gas ---> solid what is the change?
Deposition.
400
What is the symbol for heat?
ΔH
700
Draw all the phase changes