Energy that is stored.
potential
Heat transfer that only occurs in the particles of solids (and some liuids) that are touching.
conduction - closer particles, as in solids conduct faster
This is the process of changing energy from one form to another. Solar energy is changed into chemical energy by plants.
Energy transformation or conversion
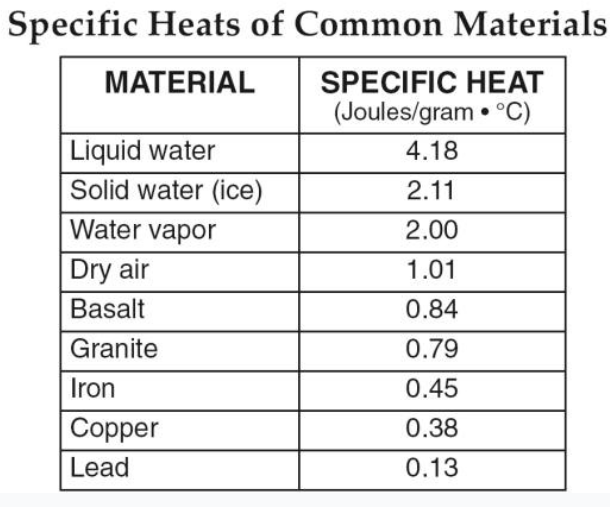
Which substance will experience the slowest temperature change?
liquid water
If you want to heat up some soup, cookware made out of which material would yield the best results?
wood aluminum
ceramic hard plastic
aluminum (it is a metal (good conductor) and has low specific heat)
States that, in any process, energy is neither created nor destroyed. It can only be converted from one form to another.
L.O.C.O.E.
This is energy of motion.
kinetic
warmer substances to cooler substances
Roller coaster moving down a hill.
PE to KE for 200 points
Gravitational potential energy to kinetic mechanical energy for 400 points
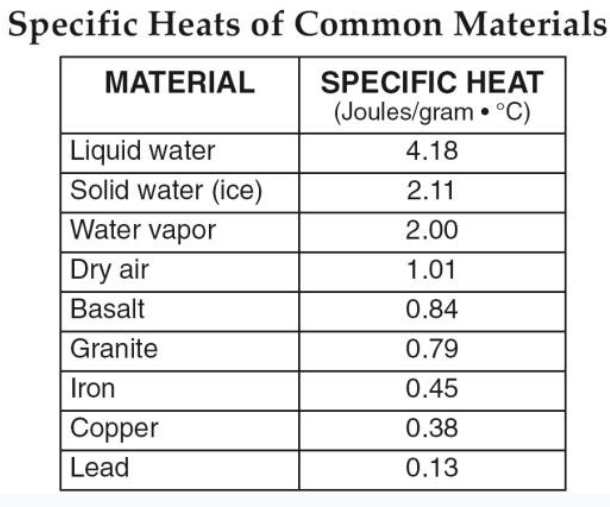
If 20kg of basalt and 20kg of granite are both heated for 10 minutes to 60 OC and then cooled to 20 OC , which one would experience the greatest temperature change over time?
granite (lower specific heat and will heat up faster)
Which phenomena is caused by convection? DOUBLE POINTS
melting marshmallows putting on gloves
weather the sun heating Earth
weather
True or false: Heat can be transferred through space via the process of conduction.
False. (no air particles in space; only radiation can be transferred there)
Type of energy found in rubber bands or crossbows.
elastic
Heat transfer that circulates warm air from the Earth's surface, and then moves it up through the upper levels of the atmosphere, and back down again.
convection
Rubbing your hands together on a cold day.
mechanical (or kinetic) to thermal
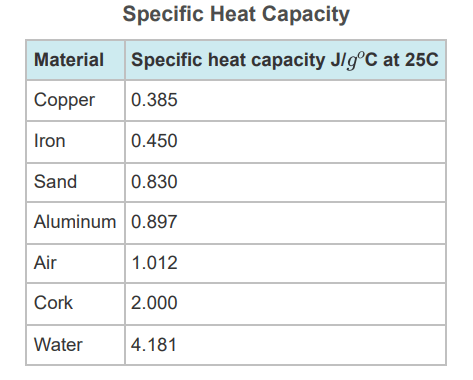
Which solid substance would make the best insulator for a metal drink container?
Cork (specific heat = 2.00 j/goC)
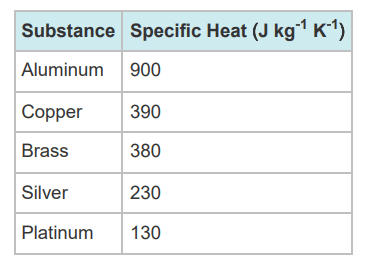
If equal masses of the 5 substances listed in this chart were heated to 200 oC, and then dropped in a bucket of 15 oC water, which one would cool down the fastest?
Platinum (it has the lowest specific heat)
What is the correct formula for calculating the joules of heat that is needed to change the temperature of a substance?
Q = mc∆T, where Q = heat energy, m = mass, and ∆T = change in temp
Energy in the form of electromagnetic radiation. Most often associated with a wavelength that is visible to the eye.
light (radiant or electromagnetic)
The average of the kinetic energy of the particles in an object.
temperature
The dc motor in a toy car.
electrical to mechanical (or kinetic)
If the amount or quantity of a substance is doubled, the amount of heat absorbed or released by that substance is _____.
also doubled
The only type of heat transfer that can occur through the vacuum of space.
The energy transferred by a force to a moving object.
mechanical
Total random kinetic energy possessed by objects in a material at finite temperature. An object that feels hot has a lot of this, while an object that feels cool as a little of this.
thermal energy
Heat transfer that creates the "greenhouse effect."
radiation
A generator producing electricity for your house during a power outage.
mechanical (or kinetic) to electrical
What is the correct formula for calculating specific heat? (DOUBLE POINTS = all of nothing)
c = Q / m x (Tf–Ti)
A 500 gram cube of lead is heated from 25 °C to 75 °C. How much energy was required to heat the lead? The specific heat of lead is 0.129 J/g°C.
Include correct units in your answer; all or nothing
Q = mcΔT
Q = (500 grams)·(0.129 J/g°C)·(50 °C)
Q = 3225 J
What mass of water will change its temperature by 3 0C when 525 J of heat is added to it? The specific heat of water is 4.184J/g°C.
m = Q = 525 J = 40 g
c x (Tf–Ti) (4.184J/g°C)(3°C)