1. What is matter?
Anything that has mass and takes up space.
2. What is a mixture?
Two or more substances PHYSICALLY combined.
3. What is a compound?
2+ elements chemically combined in a FIXED ratio.
4. What is a physical change?
A change that does not form a new substance - melting, crushing, freezing, dissolving, etc.
5. What is energy?
The ability to do work or cause a change.
6. What are the properties of a liquid?
takes up space/form of container, molecules moving, level surface
7. What type of mixture is a salad?
Heterogeneous mixture.
8. Which diagram represents a pure substance?
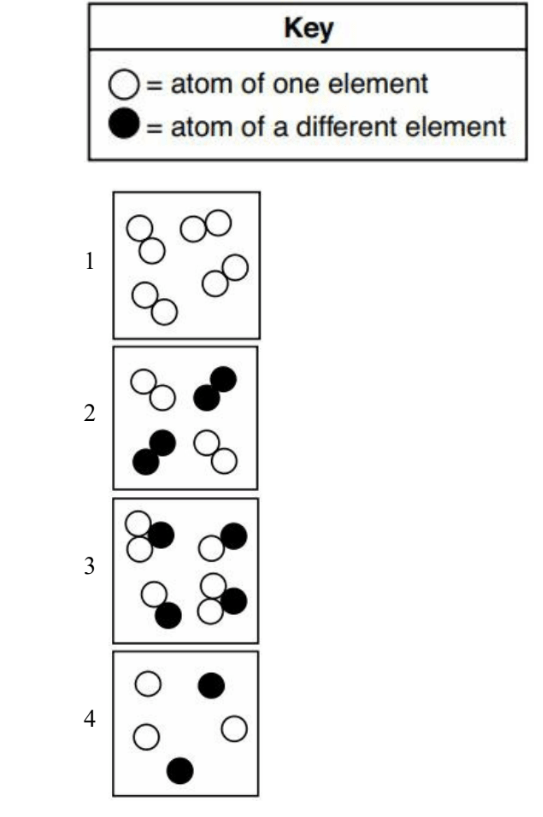
1
9. What is a chemical change?
A process that creates a new substance with different properties - combustion, rusting, digestion
10. What is conserved during a chemical reaction?
Matter and energy
11. Which particle diagram represents the solid, the liquid, and the gas?
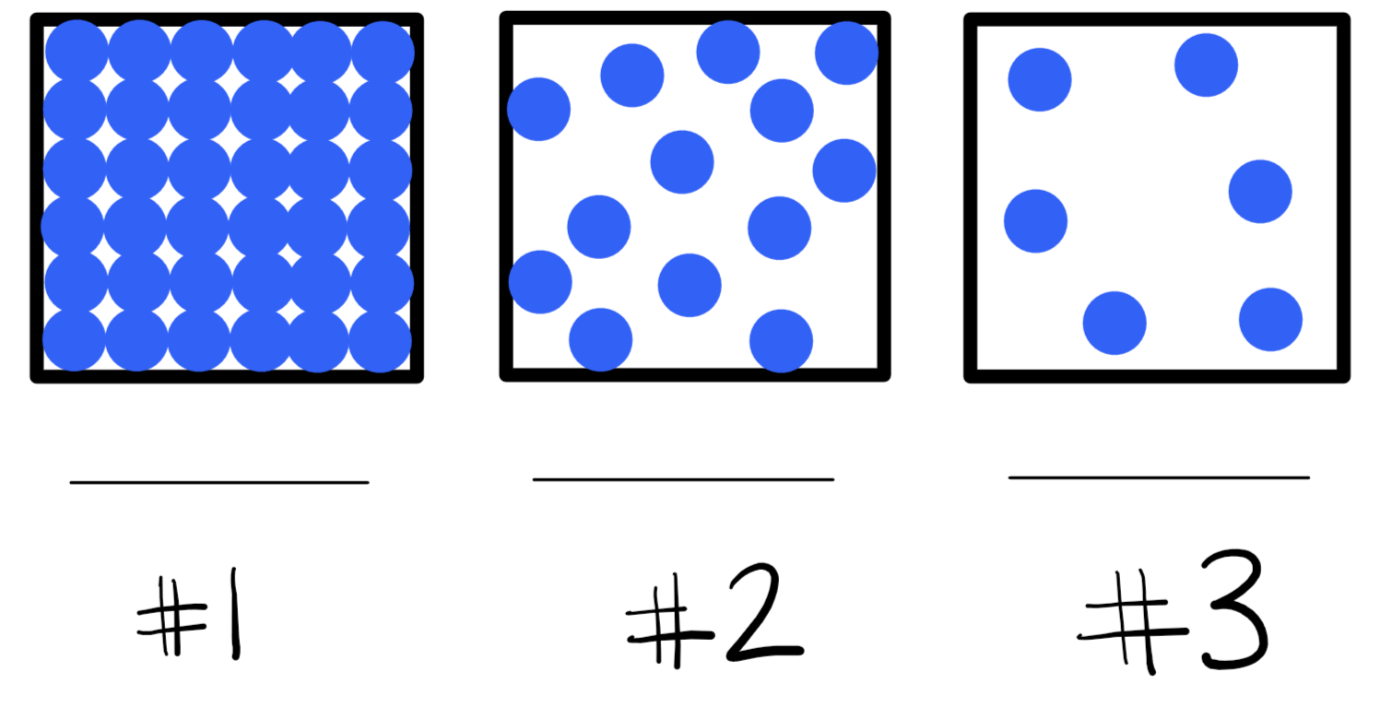
1 = solid
2 = liquid
3 = gas
12. What method would you use to separate Al(OH)3(s) from an aqueous solution?
Filtration.
13. How do we differentiate between elements?
OR
All atoms of the same element have the same ___?
All atoms of the same element have the same atomic number or number of protons. Atomic number is unique to an element.
14. Give 3 ways you'd know a chemical change was occurring:
Produce bubbles/foam/odor, colors change, sound/light/heat release, etc.
15. Describe the flow of energy when a 100 oC metal block is placed in 25 oC water in a 45 oC room.
Energy is transferred from the block to the water then to the room
16. Which two particle diagrams represent two different phases of the same compound?
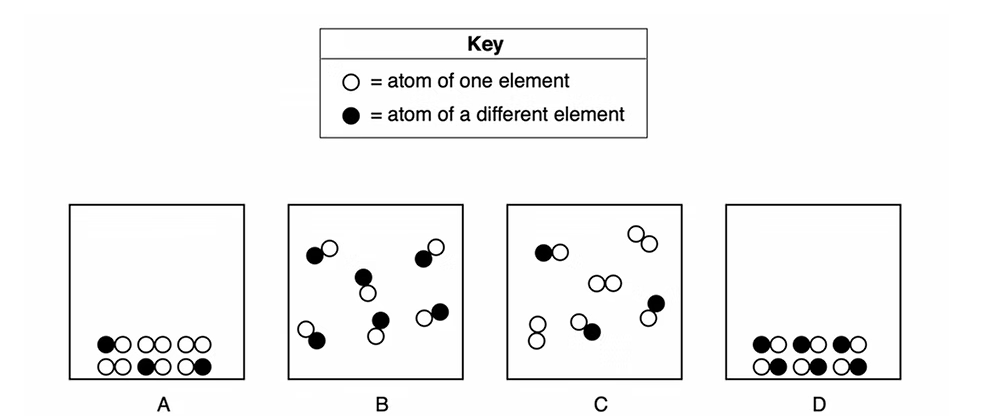
B (liquid/gas) and D (solid)
17. Which diagram represents a mixture?
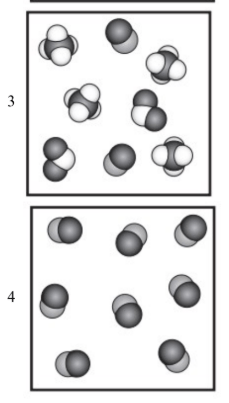
3
18. What is the difference between a compound and a molecule?
A compound describes 2+ DIFFERENT elements chemically bonded. Molecules can be any group of 2+ atoms chemically bonded together. All compounds are molecules, but not all molecules are compounds! Ex. O2 is a molecule, two atoms, but they are the same element, so it is not a compound.
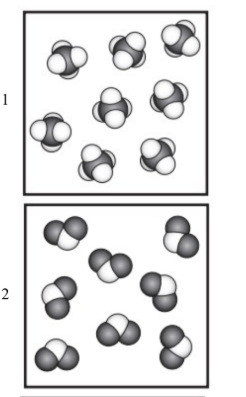
19. Which particle diagram represents a chemical change?
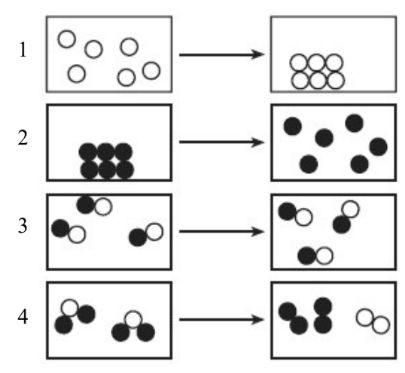
4
20. Systems move towards a state of ___________ energy.
Systems move towards a state of lower energy.
21. How does the balanced equation show the conservation of mass?
6CO2 + 6H2O -> C6H12O6 +6O2
There are the same number and type of atoms on each side.
Left: 6 C, 12 H, 18 O
Right 6 C, 12 H, 18 O
22. What is one difference between mixtures and compounds?
Mixtures are a physical combination of 2+ substances, compounds are chemically combined
Ratios of mixtures can vary, a compound has a definite ratio/composition
Mixtures can be physically separated, compounds cannot
23. Which chemical formula represents an element? H2, H2O, or Fe
H2 AND Fe
24. If I combine two substances (ie. pumping two different gases into a container), and there is no change in energy, have I created a mixture or a compound?
A mixture - if no energy change is observed, no bonds are being formed/broken. If no bonds are formed/broken, there are no compounds formed/broken. This situation describes a physical combination of two substances.
25. A 10.00 gram sample of water, initially at 25.0 oC, absorbs 515 Joules of heat when heated to some final temperature. If the specific heat of water is 4.18 J/goC, what is the final temperature of the water? Answer must have proper sig figs.
q/mc + Ti = Tf
515/(10.00 x 4.18) + 25 = 37.3 oC