standard enthalpy change of hydration?
∆H0hyd
What are the standard conditions?
Pressure of 105 Pa or 100 kPa or 1 atm
Temperature of 298K or 25 0C
Each substance involved in the reaction in its normal physical state (solid, liquid or gas) at 105 Pa and 298K.
this is symbol for the net energy change of a reaction
What is delta H

This type of reaction has heat leaving the system for the surroundings.
what is exothermic?
Standard enthalpy change of neutralization?
∆H0n
What symbol is used to show that enthalpy change is done under standard conditions?

What is enthalpy?
Enthalpy is the amount of heat in a system.
Hot packs are an example of this type of reaction.
What is exothermic?
Standard enthalpy change of reaction
∆H0r
Which standard enthalpy change is defined by the following statement:
Enthalpy change when one mole of a substance is burnt in excess oxygen under standard conditions.
Standard enthalpy change of combustion.
What is enthalpy change and how is it calculated?
Enthalpy change is the energy exchange between a chemical reaction and its surroundings at constant pressure.

This type of process is required to break bonds.
What is endothermic?
Standard enthalpy change of atomization
∆H0atm
Double Jeopardy
Write an equation to represent the standard enthalpy change of combustion of ethanol.
Standard enthalpy change of combustion of ethanol has a value of -1367.3 kJmol-1
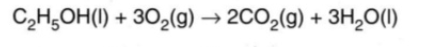
∆H0c = -1367.3 kJmol-1
If the forward reaction has an enthalpy change of -300 kJ, the reverse reaction has this enthalpy change.
What is 300 kJ?
The type of reaction could have a change in enthalpy of 400 kJ.
What is endothermic?
Standard enthalpy change of solution definition and symbol
∆H0sol is the enthalpy change when one mole of solute is dissolved in a solvent to forn an infinitely dilute solution under normal conditions.
What is standard enthalpy change of formation?
The enthalpy change when one mole of a compound is formed from its elements under standard conditions.
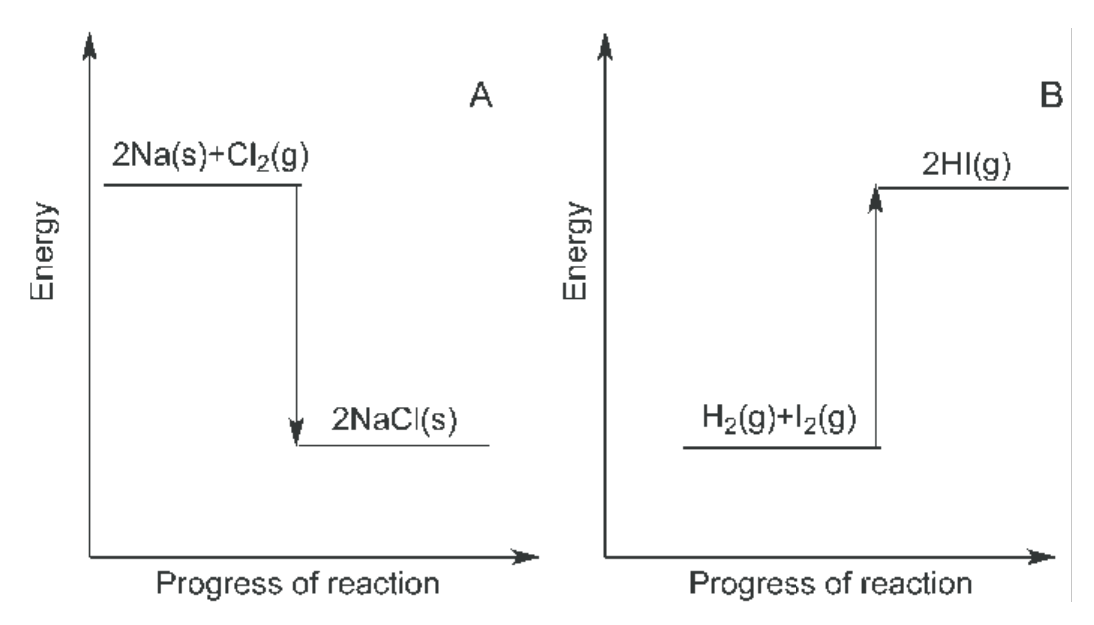
Compare the enthalpy of reactants and products in endothermic and exothermic reactions.
For an exothermic reaction the energy is released to the surroundings so the enthalpy of the reactants > enthalpy of the products.
For an endothermic reaction the energy is absorbed from the suroundings by the system so the enthalpy of the products > enthalpy of the reactants.
Double Jeopardy
Reactions can be endothermic, exothermic or ISOTHERMIC. Draw enthalpy profile diagrams for both exothermic and endothermic reactions.