__________ energy is the energy of motion, either kinetic or potential
Mechanical
What kind of energy transformation occurs on a pool deck during a hot day
What is light (radiant) energy to thermal energy
The earth is heated by the sun through what form of heat transfer?
What happens to temperature during a phase change?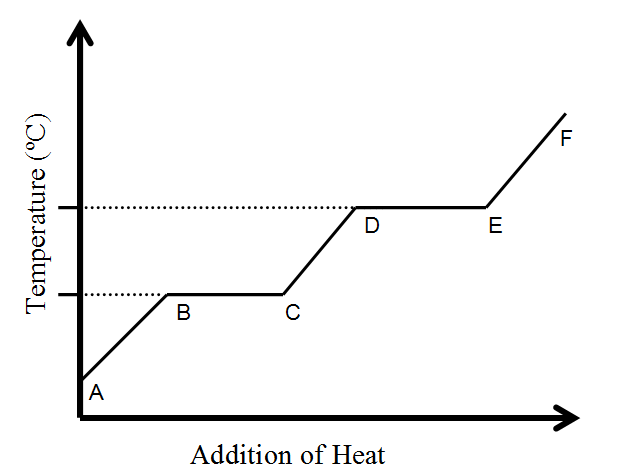
STAYS THE SAME
Specific Heat is the amount of energy it takes to increase the temperature of 1 gram of a substance by 1 degree Celsius.
What are the units for specific heat?
J/g°C
What is the energy of the motion of particles?
Thermal/Heat
Identify the START and END forms of energy:
When a nuclear reactor splits an atom to boil water
Nuclear --> thermal
Heat transfer through touch is
Conduction
Name the phase change from D --> E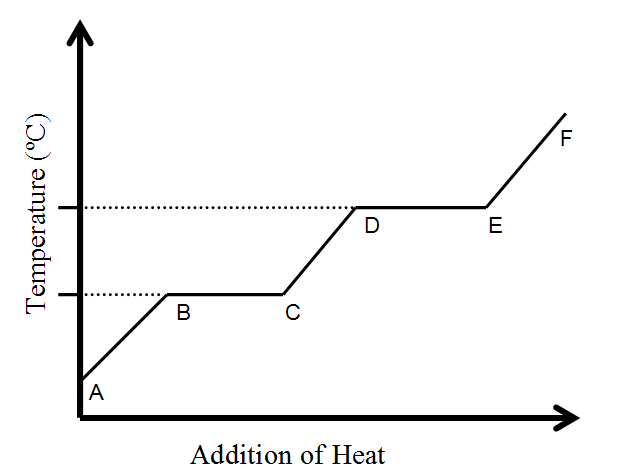
Evaporation/Boiling/Vaporization
In terms of specific heat, describe insulators vs. conductors
Insulators: High specific heat
Conductors: Low specific heat
What is the Law of Conservation of Energy?
Energy cannot be created or destroyed
Identify the START, MIDDLE and END forms of energy:
When a battery is connected to a bulb through a wire
Chemical --> electrical --> light
When something heats up, how is the movement of particles affected?
Particles move FASTER
Name a phase change that gains energy
Melting or Evaporating/boiling/vaporization
Which one do I choose if I want something that heats up quickly and cools back down quickly?
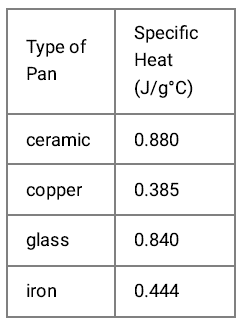
Copper (lowest specific heat)
If temperature goes down, what does that mean for the kinetic energy of particles?
Particles SLOW DOWN
When a ball rolls down a track, it has LESS kinetic energy at the bottom than the potential energy it had at the top. Where did the rest of the energy go?
LOST AS HEAT
What state of matter conducts heat the best AND WHY?
solid- particles closer together
Where is the kinetic energy of molecules INCREASING?
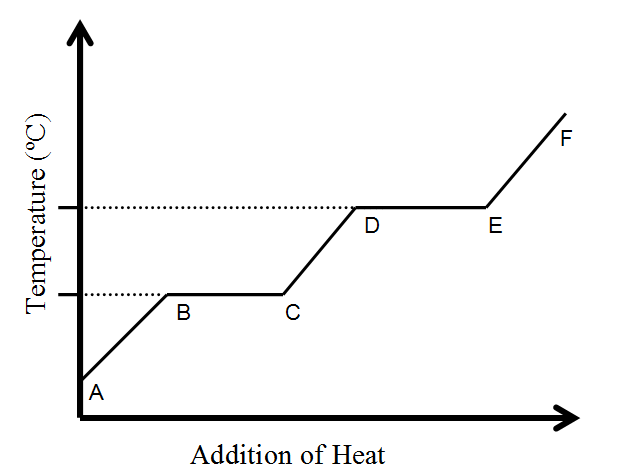
A to B, C to D, and/or E to F
I want to bake a loaf of bread, and I need a pan with a material that takes a while to heat up and will hold it's temperature for a long time.
Do I want something with a low or high specific heat?
High specific heat
Takes longer to heat up, and longer to cool down.
As a ball rolls down a hill, potential energy transforms into kinetic energy. What happens to total energy?
STAYS THE SAME
Identify the START, MIDDLE, and END forms of energy:
When a solar panel is used to power an electric fan
Light --> electrical --> mechanical (movement of blades)
Where should you put an ice pack to cool down a warm can the fastest? What type of heat transfer is this?
On TOP- cool liquid sinks, pushes warm liquid up against the ice pack
CONVECTION
Where on the heating curve is the potential energy increasing?
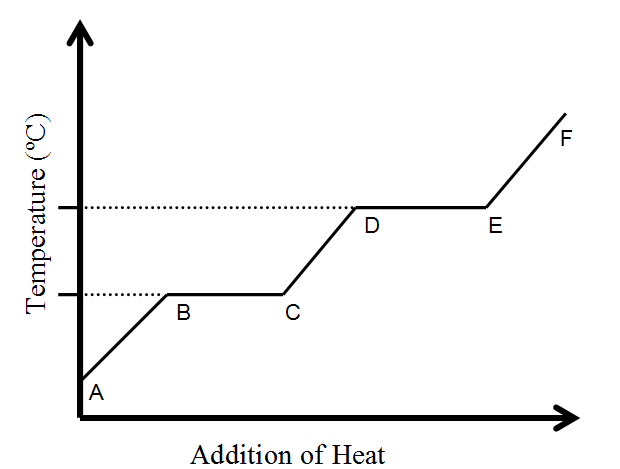
B to C AND D to E
A 50 gram piece of glass with the specific heat of 0.84 J/g°C is heated from 35°C to 46°C. How much heat is added to the glass?
(50g)(0.84J/g°C)(11°C)=
462J