Luke measured the density of water to be 0.97g/mL. Lance knew that the density of water was 1.00g/mL. What was Luke's percent error?
-3.0%
Which box is less dense?
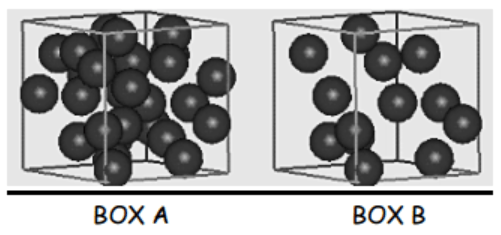
Box B
3.4mm=_______cm
0.34cm
What are these called?

beakers
What equipment was used for the Measurement Lab?
Ruler, Metric Sticks
Alora was measuring the distance between the desks to make sure they were 3.0ft apart. When she measured the desk she found that they were only 2.4ft apart. What is the percent error?
-20%
What is the definition for density?
The degree of compactness of a substance.
234.45mL=________L
0.23445L
What is this called?

Safety Goggles
What were the equipment used in the Color Lab?
Dropper, Beaker, Graduated Cylinder, Test Tubes, Test Tube Rack
Maya wanted to conduct an experiment using magnesium wire. Maya just guessed the measurement and so the magnesium wire was 9.6cm long. When Sim looked back at the directions to the experiment he found that the wire should have been only 6.5cm long. What is the percent error?
47.7%
A solid measured to be 23.5g and has a volume of 5.6cm3. What is the density of this solid?
4.2g/cm3
34g=_________mg
34000mg
What is this called?

Graduated Cylinder
What was the purpose for the Reaction in a Bag Lab?
To use the scientific method to determine which combinations of chemicals are responsible for changes when four chemicals are mixed together.
Ryan was making a pizza at Pavone's Pizza for Kyle. Ryan knew that Kyle liked the pizza sauce so he put 50mL on the pizza instead of the usual 40mL. What is the percent error?
25%
The density of a liquid is 14.5g/mL. If the volume measures to be 1.9mL, what is the mass?
27.55g
0.0345L=__________cL
3.45cL
What is this called?
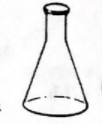
Erlenmeyer Flask
What was the purpose of the Soda Demonstration?
To see how different solutions can increase or decrease density.
Grace was doing an acid-base experiment where she needed 7.6g of the solid. Isabella had 10.7g left over from her experiment, so she gave hers to Grace. What is the percent error?
40.8%
A 78.9g irregular solid was placed into 30.0mL of water. The water then rose to 36.8mL. What is the density of the irregular solid?
11.6g/mL
34.456Km=___________mm
34456000mm
What is the measurement reading?
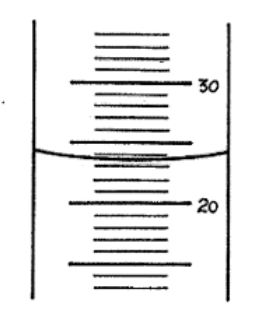
23.5 mL
What was the purpose of the Density Lab?
To understand how to obtain the data for calculating the density of liquids, a regular shaped solid, and a irregular shaped solid.