How many nanometers are in a meter?
10^9 nm
0.0102 kcal
Which of the following is a Halogen?
Mg, Mn, Li, Ne, or F
Fluorine (F)
Write this number in scientific notation:
93,000,000
9.3 x 10^7
What's the definition of a pure substance?
A type of matter with a fixed or definite composition - element or compound
8.376 x 10^-8 gigagrams
What is 479.32 Kelvin in Fahrenheit?
402.94°F
A substance is used as a semiconductor, is it most likely to be a metal, metalloid, or nonmetal?
Metalloid
This number contains how many significant figures?
207,000.
6 significant figures
Which of these isn't a chemical change?
Fireworks, boiling water and salt, digesting a donut or iron rusting?
How many inches in 2.23 km? (1 mile = 1.61 km, 5,280 ft = 1 mile, 12 in = 1 ft)
87,800 in
Label the heating curve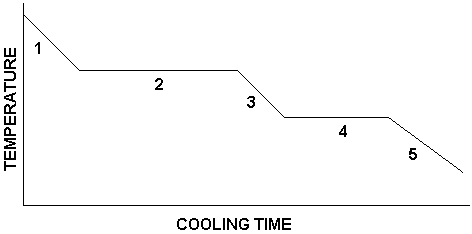
1 = gas
2 = condensation
3 = liquid
4 = freezing
5 = solid
An atom of Gallium has a mass number of 68. How many protons, electrons, and neutrons does it have?
31 protons, 31 electrons, and 37 neutrons.
Solve the math problem. Provide the answer in the correct number of significant figures:
12.5849 mg + 2.4 mg = ?
15.0 mg
What is the specific heat of a metal if 9.8g releases 40.2J of heat when the temperature is raised from 14.2K to 23.7K
0.43 J/gK
A piece of wood weighs 2.15 lbs. How much do 20 pieces of wood weigh in kilograms? (2.20 lb = 1 kg).
19.5 kg
How many Calories are in a stick of butter with 0g of carbs, 92.2g of fat, and 1.001g of protein.
Carbohydrate = 4 kcal/g
Fat = 9 kcal/g
Protein = 4 kcal/g
800 kcals
Do isotopes differ in the number of protons, electrons, neutrons, or any mix of the three?
Differ in number of neutrons (different mass numbers)
Solve the math problem. Provide the answer in the correct number of significant figures:
(7.67 cm x 3.1334 cm) / 2.775 cm = ?
8.66 cm
When water freezes, is energy absorbed or released?
Released
A doctor prescribes a dosage of 0.25 mg of a drug. Each pill contains 10 micrograms of a drug. How many pills do they need?
25 pills
How many Kilojoules are released when 13.4 g of water vapor condenses?
Heat of Fusion = 334 J/g
Heat of Vaporization =2260 J/g
30.3 kJ
2.531 amu
Solve the math problem. Provide the answer in the correct number of significant figures:
3340 ft x 1.2 ft = ?
4.0 x 10^3 ft^2
The specific heat of gold is 129 J/kg∙K. What is the quantity of heat energy (in joules) required to raise the temperature of 100.g of gold from 319.2K to 71.4°C?
326,000 J