These are the 3 levels of hybridization
T or F- sigma bonds change in between resonance structures
F- that's pi bonds
This is the makeup of alcohol
R-OH
The stronger the IMF, the ___ the melting/boiling point
higher
What is the hybridization of atom 6?

sp3
These are what are moving in resonance structures
electrons
This molecule contains which functional group
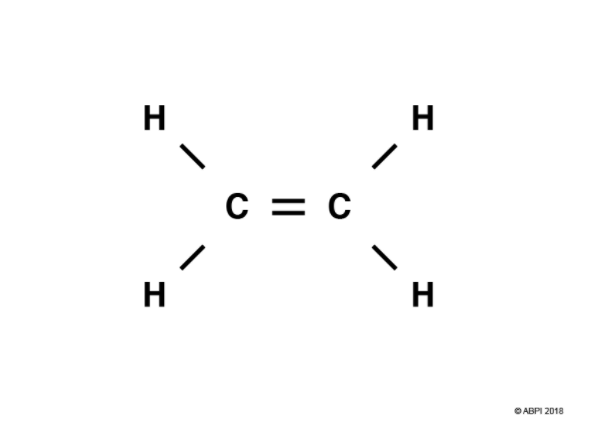
alkene
Hydrogen bonds can occur between hydrogen and these atoms
N, O, S
This is what orbital?
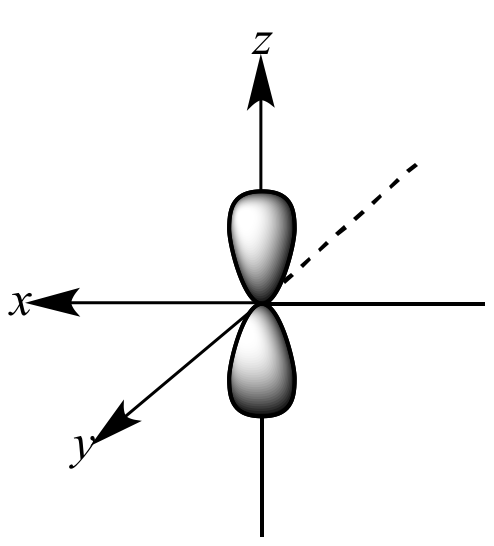
p orbital
How many electron flow arrows do we draw when moving around a positive charge? How many for a negative?
1, 2
Aside from an alkane, this molecule contains this functional group
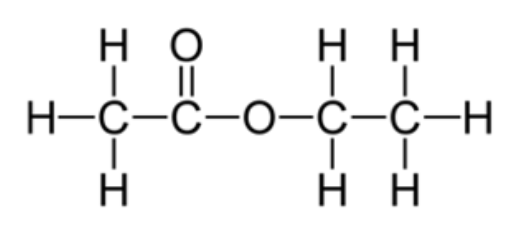
Ester
List the 4 main IMF's in order from strongest to weakest
Electrostatic interactions, hydrogen bonds, dipole-dipole, London-dispersion
What is the bond angles on atom 3?

120 degrees
Draw a resonance structure for this molecule
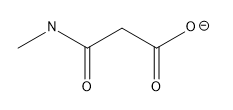
good job :)
What is the makeup of an alkyl halide
C-X (F, Cl, Br, I)
Where would the polarity arrow be in this image
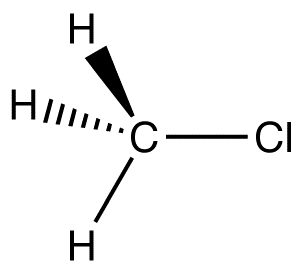
Pointing from C to Cl
These are the 3 geometries we discussed in class
linear, trigonal planar, tetrahedral
Draw the hybrid structure for these resonance structures (one hybrid for them all)

good job :)
List ALL functional groups present
alkane, benzene ring, amide, carboxylic acid
What is the "Rule of 5" in water solubility
Everyone -OH or -NH can have around 5 carbons attached and still be water soluble