What is the molar mass of water (H₂O)?
Answer: 18.02 g/mol
What is the formula for percent composition?
mass of chosen element
---------------------------- x 100
total mass of compound
What is the saying that helps us remember the last steps to molecular formula? Explain
"DON'T FORGET ABOUT M/E"
We are dividing the mass of the Molecular formula and the Empirical formula
Describe the formula for Coulomb’s Law
The force between charged particles increases (Is proportional) with charge and decreases with distance (because the radius is INVERSLEY proportional to force).
Which element has the largest atomic radius: Li, Na, or K?
Answer: K
* Draw the molar mass triangle
How many moles are in 45.0 g of NaCl?
Answer: 0.78 mol
What does “percent composition” tell you about a compound?
Answer:
It tells you the percentage by mass of each element in a compound.
When calculating an empirical formula, what should you do if one of your subscripts ends in .5 (like 1.5 or 2.5) instead of a whole number?
Answer: Multiply all subscripts by 2 to make them whole numbers.
According to Coulomb’s Law, as distance between particles increases, the force of attraction ___.
Answer: Decreases.
Which element has the highest ionization energy:
C, N, or O?
O
0.5 moles of sodium chloride (NaCl) is dissolved to make 0.05 Liters of solution. Find the Molarity
Answer: 10M
What percent of hydrogen is in water (H₂O)?
Answer: 11.1%
A compound has an empirical formula of CH and a molar mass of 78 g/mol. Find the molecular formula.
Answer: C₆H₆
In a PES spectrum, the position of the peak represents.....
The binding (ionization) energy required to remove the electron from that particular subshell
What happens to electronegativity across a period (left to right)?
Answer: It increases.
What is the molarity of a solution made by dissolving 10.0 g of NaOH in 250.0 mL of solution?
Answer: 1.0 M
A compound has the formula C₆H₁₂O₆. What is the percent composition of carbon?
Answer: 40.0%
Find the empirical formula for a compound with 52.2% C, 13.0% H, and 34.8% O.
Answer: C₄H₁₁O₂
What is the formula if Boron (B) bonded with Sulfur (S)?
B2S3
Explain why He has an electronegativity value of 0
He is a noble gas, meaning that their octet is full (not wanting to share electrons nor attract)
*Draw both Molarity and molar mass triangles
How many grams of solute are in 750.0 mL of a 0.500 M Na₂SO₄ solution?
Answer: 53.3 g
A popular candy bar has the following nutrition facts per serving (50.0 g total):
Fat: 12.0 g
Protein: 3.0 g
Carbohydrates: 10.0 g
Sugar: 24.0 g
Calculate the percent composition by mass of fat in the candy bar.
Answer: 24%
What’s the difference between an empirical and a molecular formula?
Empirical = simplest ratio
Molecular = actual number of atoms.
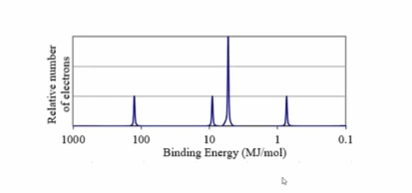
What is the element and it's configuration?
Magnesium
1s22s22p63s2
Explain why Francium (Fr) has one of the lowest ionization energies in respect to Coulomb's Law
Francium’s outer electron is farther from the nucleus (q1 & q2), so the attraction is weak (since force ↓ as distance ↑). It takes little energy to remove it.