What is the effect of enzymes on equilibrium?
Enzymes do not affect the equilibrium of a chemical reaction. Instead, they accelerate the rate at which equilibrium is reached by lowering the activation energy required for the reaction to proceed.
What is the catalytic triad?
The catalytic triad is a group of three amino acids found in the active site of some enzymes that work together to catalyze the cleavage of peptide bonds during proteolysis. The triad typically consists of serine, histidine, and aspartate; these residues are arranged to enable the enzyme to break down proteins efficiently.
Describe T and R state
- T state: Low-affinity, more rigid, favored in low-ligand conditions.
- R state: High-affinity, more flexible, favored in high-ligand conditions.
In proteins like hemoglobin, the equilibrium between these two states is key to their ability to bind and release oxygen efficiently based on environmental needs.
A ligand example is oxygen.
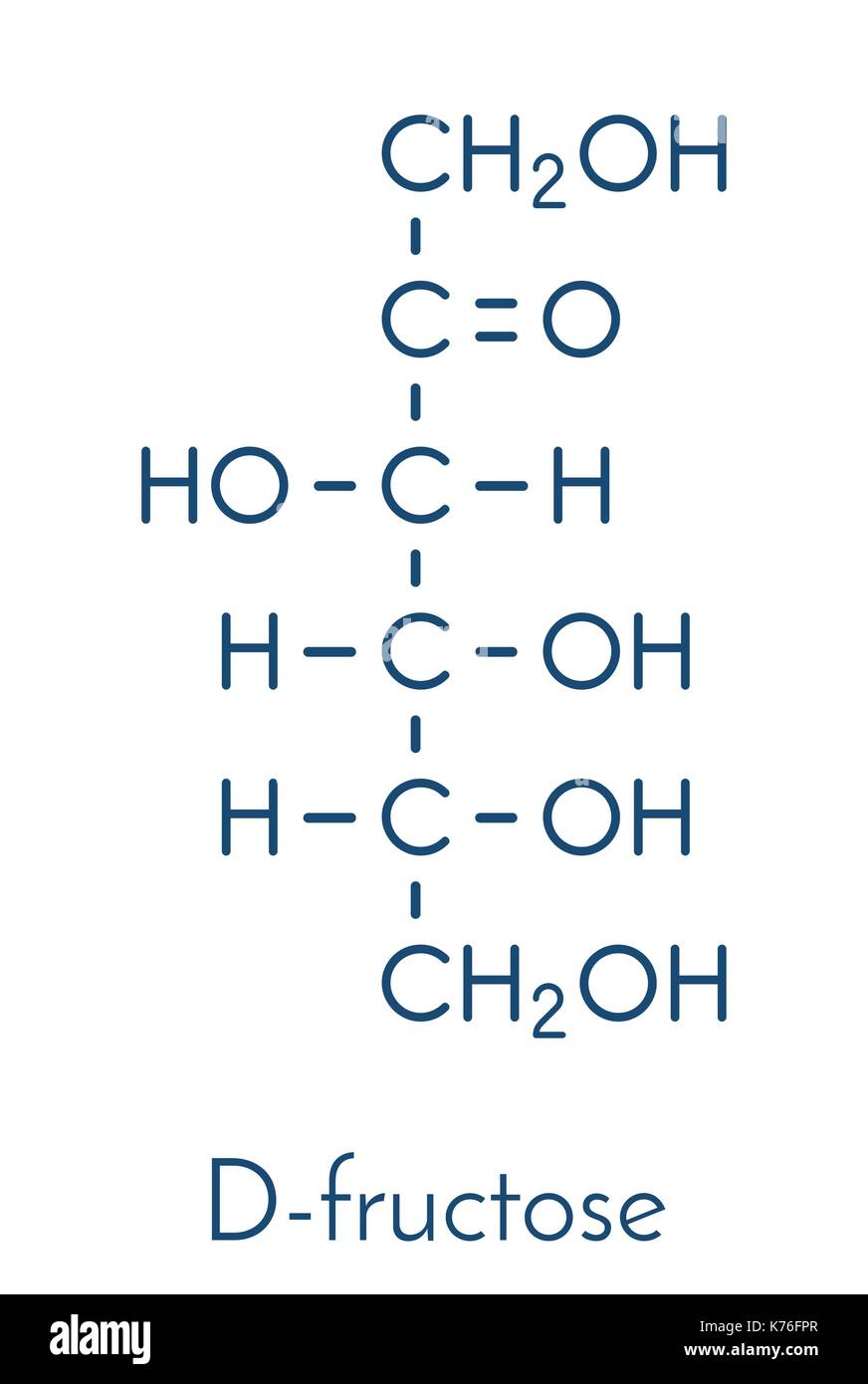
Draw fisher projections for Glucose, Galactose, and Fructose
What is the general structure of a phosphoglyceride?
glycerol backbone, phosphate, two fatty acid tails, and a head group
What’s the difference between Q and Keq?
Keq is the ratio of the concentrations of products to reactants when a reaction has reached equilibrium, where the forward and reverse reactions occur at the same rate.
Q is the ratio of the concentrations of products to reactants at any point during a reaction that has not necessarily reached equilibrium.
- If Q<Keq, the reaction will proceed forward (toward products).
- If Q>Keq, the reaction will proceed backward (toward reactants).
- If Q=Keq, the reaction is at equilibrium.
In carbonic anhydrase, this metal ion plays a critical role in stabilizing the water molecule for proton transfer.
Zinc
What is the primary function of protein kinases in cellular signaling?
A. To remove phosphate groups from proteins, decreasing their activity
B. To add phosphate groups to specific amino acids, increasing the activity of enzymes
C. To degrade proteins into amino acids
D. To synthesize proteins from amino acids
B. To add phosphate groups to specific amino acids, increasing the activity of enzymes.
Explanation:
Protein kinases catalyze the addition of phosphate groups to serine, threonine, or tyrosine residues on proteins, which typically enhances the activity of the enzymes by lowering their Km and/or increasing their kcat, thus playing a crucial role in cellular signaling.
What distinguishes an aldose from a ketose?
A. Aldoses have a carbonyl group at the end of the carbon chain, while ketoses have it in the middle.
B. Aldoses contain a hydroxyl group, while ketoses do not.
C. Aldoses have a higher molecular weight than ketoses.
D. Ketoses are more soluble in water than aldoses.
A. Aldoses have a carbonyl group at the end of the carbon chain, while ketoses have it in the middle.
Draw the structure of a phosphoglyceride, labeling the glycerol backbone, fatty acid tails, phosphate group, and head group. Explain the significance of the head group in determining the membrane properties.
The head group influences membrane fluidity, permeability, and interactions with proteins. Different head groups can lead to variations in membrane curvature and the formation of lipid rafts.
Which of the following does an enzyme bind to with the strongest affinity?
a. substrate
b. product
c. transition state
d. enzymes must bind to all of the above with the same affinity
c. transition state
Enzymes are designed to bind to the transition state of a reaction with the highest affinity. This is because the enzyme stabilizes the transition state, lowering the activation energy required for the reaction to proceed. By binding more tightly to the transition state than to the substrate or product, enzymes effectively facilitate the conversion of substrate into product, enhancing the reaction rate.
What’s the role of the different histidines in CA? What’s the role of Zn?
The role of the histidines are:
- After water is deprotonated by the zinc ion, His helps transfer the proton out of the active site to the surrounding environment, allowing the enzyme to reset for another catalytic cycle.
-hold the zinc ion in place
The zinc ion plays a crucial role in activating water for nucleophilic attack
What is the role of allosteric sites in enzyme regulation?
A. They bind substrates and increase the reaction rate.
B. They bind regulatory molecules that change the enzyme's activity.
C. They are identical to the active site and compete with substrates.
D. They are responsible for the enzyme's stability under heat.
B. They bind regulatory molecules that change the enzyme's activity.
Which of the following describes the Haworth structure of β-D-glucose?
A. The anomeric hydroxyl group is oriented downward.
B. The anomeric hydroxyl group is oriented upward.
C. It is in the open-chain form.
D. It cannot form a cyclic structure.
B. The anomeric hydroxyl group is oriented upward.
Compare and contrast saturated, mono-unsaturated, and polyunsaturated fatty acids in terms of their structural properties and physical characteristics. How does the degree of saturation affect the melting point of these fatty acids?
Saturated fatty acids have no double bonds, resulting in straight chains that pack tightly together, leading to higher melting points. Mono-unsaturated fatty acids have one double bond, introducing a kink that prevents tight packing, resulting in lower melting points. Polyunsaturated fatty acids have multiple double bonds, which further disrupt packing and lower melting points compared to saturated and mono-unsaturated fatty acids.
Given:
- Vmax=100 µmol/min
- Km=10 mM
- KI=2 mM
- v0=20 µmol/min
- [S]=5 mM
Calculate the concentration of the competitive inhibitor [I].
Answer Choices
A. 10 mM
B. 25 mM
C. 47 mM
D. 100 mM
- Rearranging:
(1+[I]/KI)=((Vmax⋅[S])/v0)−[S]/Km
- Plugging in values:
(1+[I]/2)=((100⋅5)/20)−(5/10)
- Calculate:
(100⋅5)/20=25 and 5/10=0.5
- Substitute back:
(1+[I]/2)=25−0.5=24.5
- Isolate [I]/2:
[I]/2=24.5−1=23.52
- Solve for [I]:
[I]=23.5×2=47 mM
Draw the second intermediate of the serine protease mechanism.
Answer on the board.
In the context of feedback inhibition, how does CTP influence the activity of ATCase in the production of pyrimidines?
A. CTP acts as an activator, increasing the enzyme's activity.
B. CTP acts as an inhibitor, stabilizing the T state and decreasing the enzyme's activity.
C. CTP has no effect on ATCase, as it only affects purine synthesis.
D. CTP directly alters the active site of ATCase to enhance substrate binding.
B. CTP acts as an inhibitor, stabilizing the T state and decreasing the enzyme's activity.
Given the following disaccharide, D-maltose (D-glucopyranosyl-(α1→4)-D-glucopyranose), predict the impact on the properties of the disaccharide if the glycosidic bond were altered to β(1→4). Which of the following statements is true?
A. The new disaccharide would have a significantly higher solubility in water.
B. The new disaccharide would be more resistant to enzymatic hydrolysis by maltase.
C. The new disaccharide would still be classified as a reducing sugar.
D. The new disaccharide would have a lower melting point than maltose.
B. The new disaccharide would be more resistant to enzymatic hydrolysis by maltase.
Explanation: The change from an α(1→4) to a β(1→4) glycosidic bond alters the enzyme specificity. Maltase, which hydrolyzes maltose, is specific for α-glycosidic bonds and would not effectively act on the β-glycosidic bond, resulting in increased resistance to hydrolysis.
Explain the hydrophobic effect and its role in the spontaneous formation of lipid bilayers in aqueous environments. What types of lipids favor bilayer formation versus micelle formation?
The hydrophobic effect refers to the tendency of non-polar substances to aggregate in water to minimize their exposure to water. Amphipathic lipids, which have both hydrophobic and hydrophilic regions, favor bilayer formation due to their dual nature, while detergents (single-tailed lipids) favor micelle formation due to their inability to form stable bilayers.
An enzyme-catalyzed reaction exhibits Michaelis-Menten kinetics. The enzyme has the following kinetic parameters:
- Vmax=200 µmol/min
- Km=5 mM
- A competitive inhibitor is present with a KI=3 mM.
At a substrate concentration [S]=10 mM and a concentration of inhibitor [I]=1 mM, calculate the initial reaction velocity v0.
- Using the Michaelis-Menten equation, what is the initial reaction velocity v0?
Answer Choices:
A. 80 µmol/min
B. 100 µmol/min
C. 120 µmol/min
D. 160 µmol/min
C. 120 µmol/min
Draw the carbonic anhydrase mechanism.

Isozymes are enzymes that catalyze the same reaction but differ in structure and regulatory properties. Which of the following statements is true regarding isozymes?
A. Isozymes are always expressed in the same tissues and at the same levels.
B. Isozymes can have different kinetic properties, allowing for regulation in various physiological contexts.
C. Isozymes have identical amino acid sequences but differ in their binding affinity for substrates.
D. Isozymes are formed from the same gene through alternative splicing.
B. Isozymes can have different kinetic properties, allowing for regulation in various physiological contexts.
Define the term "reducing sugar." How was this concept historically applied in the diagnosis of diabetes through the examination of urine samples?
Answer Choices:
A. A reducing sugar is a sugar that has a free aldehyde or ketone group, which can reduce other compounds. Historically, urine samples containing reducing sugars like glucose were tested to diagnose diabetes because elevated levels indicated hyperglycemia.
B. A reducing sugar is a sugar that can react with iodine to form a complex. Historically, urine samples were tested for reducing sugars using a colorimetric assay to diagnose diabetes.
C. A reducing sugar is any carbohydrate that can be hydrolyzed into simpler sugars. Historically, urine samples were tested for reducing sugars to monitor blood sugar levels in patients with kidney disease.
D. A reducing sugar is a sugar that has been chemically modified to enhance its reactivity. Historically, urine samples were tested for reducing sugars to determine the presence of carbohydrates in the diet.
A. A reducing sugar is a sugar that has a free aldehyde or ketone group, which can reduce other compounds. Historically, urine samples containing reducing sugars like glucose were tested to diagnose diabetes because elevated levels indicated hyperglycemia.
Explanation: Reducing sugars, such as glucose, have free aldehyde or ketone groups that can reduce other substances. In the past, the presence of these sugars in urine samples was an important indicator of diabetes, as their elevated levels suggest poor blood sugar regulation (hyperglycemia).
Discuss the role of cholesterol in cell membranes. How does it affect membrane fluidity and permeability at different temperatures?
AND
Given the following fatty acid notation, 18:2 (Δ9,12), explain its significance. What does each part of this notation indicate?
Cholesterol acts as a fluidity buffer in cell membranes. At high temperatures, it stabilizes the membrane by reducing mobility, while at low temperatures, it prevents tight packing of phospholipids, thus maintaining fluidity. Cholesterol also decreases permeability to small polar molecules.
AND
The notation 18:2 (Δ9,12) indicates that the fatty acid has 18 carbon atoms and two double bonds. The Δ9,12 indicates that these double bonds are located between the 9th and 10th carbon and the 12th and 13th carbon, respectively, counting from the carboxyl end.