Identify the central atom in the following molecules respectively: HNO, SO2, CHCl3, MgBr2, Cl2O
N, S, C, Mg, O
Order the following in increasing electronegativity: Se, Ag, O, Re, Zr, Cl
Zr, Re, Ag, Se, Cl, O
What is the molecular shape of SO2?
Bent/V-shaped
How many sigma bonds does ethylene (C2H4) contain?
5
Describe when gloves should be replaced during a lab.
Gloves should be replaced when they are contaminated, permeated by a solvent, torn/have holes, or they have been used for 2 or more hours.
How many electrons should be considered for the Lewis Structure of NO3-? Identify the central atom and determine how many resonance structures NO3- has.
24 electrons; N; 3 resonance structures
What is the electronegativity (ΔEN) of KO3? What type of bond is it?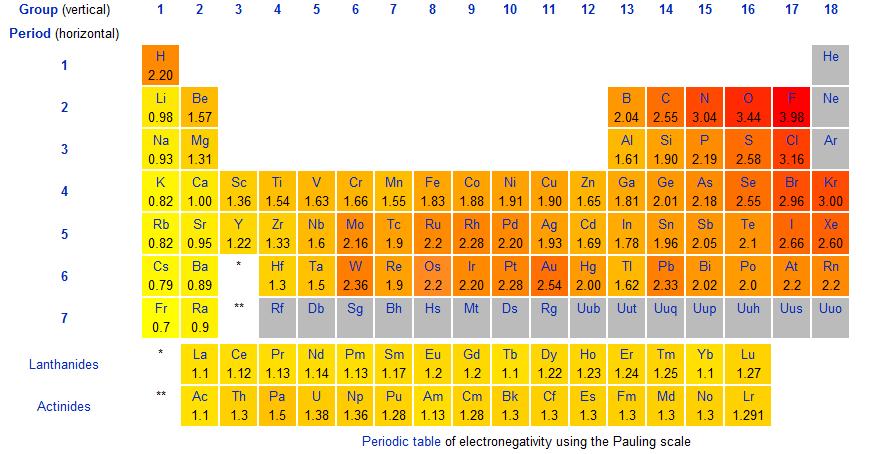
ΔEN(KO3) = 2.6, Ionic Bond.
What is the name for line-dash-wedge notation? What is it used for?
Natta notation, indicates 3D structural features in Lewis notation.
If BeF2 has a linear shape what is the hybridization around the Be atom in BeF2?
A) sp
B) sp^2
C) sp^3
A) sp
What are the two major industrial pollutants?
SOx &NOx
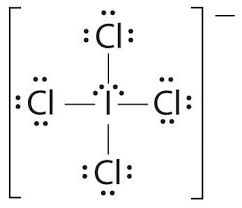
Draw the correct lewis structure for ICl4-
Order the following in terms of increasing atomic radius: K+, Ca2+, Ar, Cl-
Ca2+ < K+ < Ar < Cl- ; Ca2+, K+, and Ar are isoelectric however Ca2+ has more protons than K+ and Ar making the radius smallest. K+ is smaller than Ar as it hold more of a positive charge. Ar is smaller than Cl- due to basic size trends as Cl is already larger than Ar being to the left of it on the periodic table. The additon of an electron to Cl makes its radius even larger.
What is the electron geometry, molecular shape, and bond angle for ClF3?
trigonal bipyramidal, T-shaped, <90
 What type of bond does this image display? How do you know?
What type of bond does this image display? How do you know?
Sigma bond; it is linear along the bond axis/there is no node along the bond axis/if you look head on at the bond axis, it will look like an s-orbital (sphere)
The following GHS hazard label is used to inform users that they may be exposed to ___. 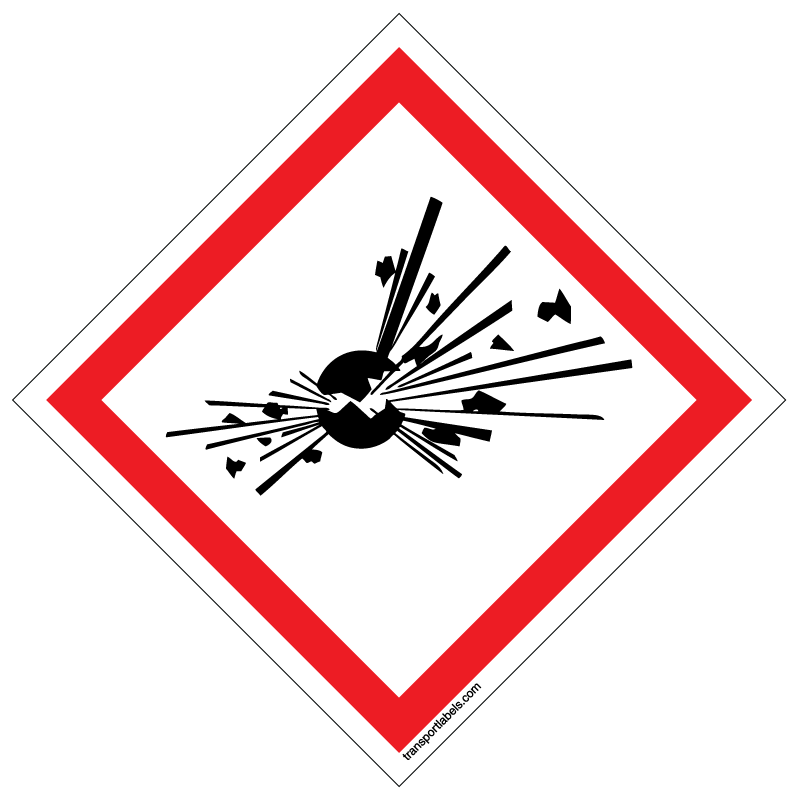
Explosives, self reactives, or organic peroxides
Draw the correct Lewis Stucture for tellurium tetrachloride.

Order the following in increasing ionization energy: N, O, B, Be, Rb, Mg
Rb < Mg < B < Be < O < N
List in order of increasing of bond angle: CO2, NH3, XeF4,NO3^-
XeF4(90)<NH3 (109.5)<NO3^-(120) <CO2 (180)
If an atom has 5 electron densities in a trigonal bipryamidal arrangment including lone pairs, single bonds, and double bonds, what is the hybridization of that atom?
sp3d
How are NO and NO2 produced?
They are air pollutants generated by the reaction of N2 during fuel combustion (smog). OR Emission from agricultural soils.
Compare the lewis structures A, D, and E for nitrous oxide. Which has the best structure? Explain why the other two are wrong. 
D; A is incorrect because the negative charge should be on the most electronegative atom. E is incorrect because the leftmost N is not satisfied by the Octet Rule.
Which bond angle is smaller: that of Ammonia (NH3) or of Arsine (AsH3)?
Arsine (due to the larger atomic radius)
What are the molecular geometry and angle for a compound which has 4 electron domains, and 3 of them are bonding electron domains and only one is a non-bonding pair of electrons? Give an example!
Trigonal pyramidal, 107,3°, NH3
Give the hybridizaion for SF4, ICl4^-, NO2-
sp^3d, sp^3d^2, sp^2
Name 3 reasons why Standard Operating Procedures (SOP) are needed
Any 3 from: Consistency in training, Safety standards, Liability, Satisfy requirements, Valuable skill for employment