What kind of bond does NaCl form? What kind of intermolecular force does it have (if any)?
It forms an ionic bond. Ionic bonds don't have IMFs.
.000400
What is 3?
1.45 + 20.3 =
What is 21.8?
63.00/13.243 =
What is 4.757?
Why does solid water float in liquid water? Draw a picture to explain your ideas. (90 seconds)
Solid water floats in liquid water because when water freezes, it's hydrogen bonds connect in a 6 sided shape. This causes the water to expand, lowering the density and allowing ice to float.
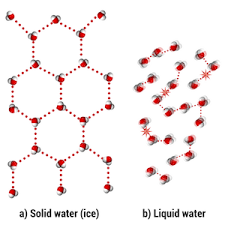
Polar covalent. Dipole-dipole.
14.06000
What is 7?
901.456 + 32.1895 =
What is 933.646?
2.59 x 3.0 =
What is 7.8?
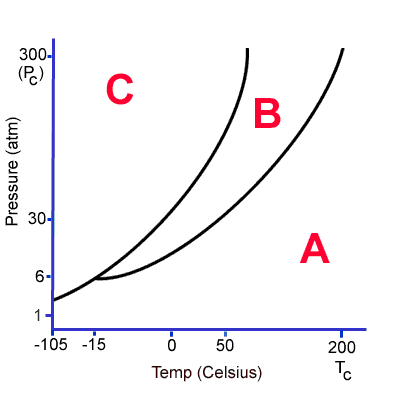
At 0C and 100 atm, what state of matter is this substance? How could I change it?
It is a solid.
I could change it to a liquid by increasing temperature. If I continued to increase temperature it would change into a gas.
I could lower pressure and it would become a gas.
Draw a dipole-dipole between two molecules. Make sure those molecules form the right type of bond that would allow a dipole-dipole to exist.
Answers will vary
304,000.
What is 6?
12 + 14.34 =
What is 26?
.0046/23.4 =
What is .00020? or 2.0 x 10-4?
Substance A has a boiling point of 100C. Substance B has a boiling point of 20C. Which has a stronger intermolecular force?
Substance A since it takes more energy to break the intermolecular force.
Classify bond type and intermolecular force for the following:
H2O
O2
PO2
LiBrH2O - Polar covalent. Hydrogen Bond
O2 - Nonpolar covalent. Dispersion forces.
PO2 - Polar Covalent. Dipole-Dipole
LiBr - Ionic
1.0
What is 2?
29.0 - 14.83 =
What is 14.2?
20 x 4.56 =
What is 90?
What happens when a salt (CaCl) dissolves in water? Draw a picture to explain your idea.
The ionic bond temporarily breaks since the ions are more attracted to the positive and negative side of water.
Why is a dipole-dipole attraction stronger than a dispersion force?
A dispersion force is a temporary attraction between two molecules, whereas the dipole-dipole is a permanent attraction due to differences in electronegativity. Because the dispersion force "comes and goes" it is weaker.
4.560 x 103
What is 4?
7.058 x 1036 - 5.3 x 1036 =
What is 1.8 x 1036?
(8.45 x 1032) x (4.3 x 10-29)
Draw a phase diagram for a fake substance. Use degrees Celsius for your temperature and atmosphere for your pressure. Label the following: solid, liquid, gas, triple point, critical point.
Answers will vary.