What are the 3 basic types of Matter?
What are solids, liquids, and gases.
Pure substances are broken down into these 2 areas.
What are elements and compounds.
These are the two main types of mixtures.
What is homogenous and heterogeneous?
This diagram represents this type of matter.

What is an element.
Ice melting is an example of this.
What is a physical change.
What is anything that has mass and takes up space?
What is matter.
Pure substances cannot be separated into simpler substances by this type of means.
What is physical means.
A mixture that has a uniformed composition would be classified as this.
What is a homogeneous mixture.
This diagram represents this type of matter.
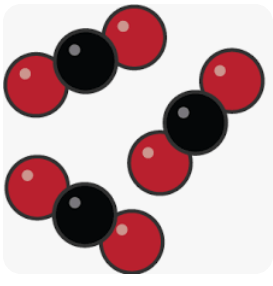
What is a compound.
A change in which a new substance is formed.
What is a chemical change.
I am this type of matter that has definite shape and volume.
What is a solid.
Elements are represented by one of this type of letter.
What is a capital letter.
This technique would be able to separate a mixture that has an uneven composition consisting of a solid and a liquid.
What is filtration.
This diagram represents this type of matter. (be specific) 
What is a mixture of diatomic elements.
Hardness would represent this property.
What is physical property.
I am this type of matter with only definite volume.
What is a liquid.
Compounds differ from mixtures because they are in this type of ratios that are represented by subscripts.
What are fixed ratios.
Solubility is the physical properties of this separation technique of two or more different liquids in a homogenous mixture.
What is chromatography.
This diagram represents this type of matter. (be specific)

What is a heterogenous mixture.
The ability to react with other substances would represent this property.
What is a Chemical Property.
This property is only for gases due to its lack of having definite shape and volume.
What is compressible.
These are the 7 diatomic elements that have a particle diagram like this
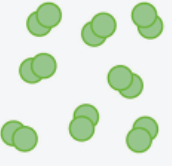
What are
Br2 I2 N2 Cl2 H2 O2 F2
These separation techniques are used because of the physical property of boiling points.
What is evaporation and distillation.
This diagram represents this type of matter. (be specific)
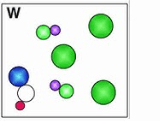
What is a mixture of elements and compounds.
This picture represents this type a change.

What is a chemical Change.