Describe fission vs fusion
In fusion, two small nuclei combine to form a larger nucleus
In fission, a larger nuclei is split into smaller nuclei and releases energy
Draw the Lewis structures for CCl4, name the electron and molecular geometry
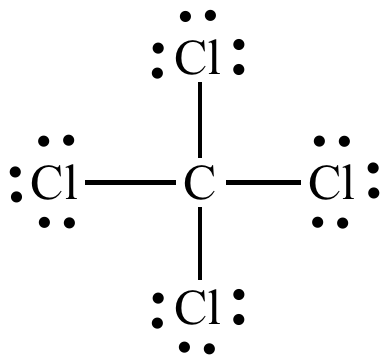
tetrahedral
list the intermolecular forces
dipole–dipole attractions, hydrogen bonding, dispersion forces
Is the bond between HBr polar or nonpolar
polar
How many moles of water, H2O, contain 2.60 × 1023 molecules of water?
0.432 mol
Why is CCl4 nonpolar?
What is the shape of the molecule is tetrahedral, so all of the polar bonds cancel each other out, and the overall molecule is nonpolar
Draw the Lewis structures for H2S, name the electron and molecular geometry

electron - tetrahedral
molecular - bent
Indicate the major type of intermolecular forces in each of the following:
H2S, H2O
dipole-dipole, hydrogen
Determine if the following would be polar or nonpolar: Cl2
nonpolar (only nonpolar bonds)
How many Li atoms are in 4.5 moles of Li
2.71*1023 atoms
Which element has the highest ionization energy in the D block?
gold
Draw the Lewis structures for Cl2O, name the electron and molecular geometry
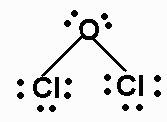 electron- tetrahedral
electron- tetrahedral
molecular - bent
Indicate the major type of intermolecular forces—dipole–dipole attractions, hydrogen bonds, or dispersion forces—expected of each of the following:
HF, Br2, PCl3
hydrogen, dispersion, dipole-dipole
Determine if the following would be polar or nonpolar: SiCl4
nonpolar (polar covalent bonds cancel each other out)
Calculate the mass, in grams, of 1.50 moles of Na
34.5 g
What block on the periodic table contains most of the nonmetals
The P block
Draw the Lewis structures for BH4-, name the electron and molecular geometry

tetrahedral
identify which has the strongest intermolecular forces between the particles:
CH3OH
CO
CF4
CH3CH3
CH3OH - hydrogen bonding
Determine if the following would be polar or nonpolar:
PCl3
polar (all polar covalent bonds that don't cancel out)
Which has a higher boiling point: HF or H2O, Why do you think this is?
H2O because the intermolecular bonds are able to line up better into an stronger form. H-F has three negative areas and one positive area. Water is 2 and 2.
What does VSEPR stand for? Explain VSEPR in your own words.
What is valence-shell electron-pair repulsion? What is the valence electrons in atoms that are bonded to each other repel each other and try to get as far away from each other as possible - this determines the shape of the molecule.
Draw the Lewis structures for H2CO (C= central atom), name the electron and molecular geometry

trigonal planar
Identify the substance that would have a higher melting point and explain your choice:
CH4 or CH3OH
CH3OH - hydrogen bonding compared to dispersion forces
Determine if the following would be polar or nonpolar:NF3
polar (polar bonds don't cancel out net polarity in one direction)
What is a covalent substance that has atoms bonded to a central halogen?
Xenon Tetraflouride