Organic Chemistry (each question worth double points)
Organic Chemistry (each question worth double points)
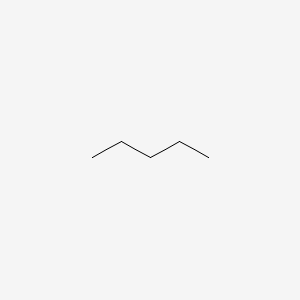
The IUPAC name for the following compound:
Pentane
The phase state of this substance:
NaNO3
Aqueous
Sodium Nitrate is soluble in water
mol/L
Of the two following electromagnetic waves, this one has the greater frequency:
X-ray or Visible
X-rays
This is the formula that gives the number of possible values for ml given the l value
2(l) + 1
-l ≤ ml ≤ l
The name of the following compound:
Benzene
The phase state of this substance:
SrSO4
solid
Strontium Sulfate is insoluble
The concentration of a sodium chloride solution given I have 50 grams in 250 mL
(answer with 3 decimal places)
50 grams NaCl = 0.85557 moles NaCl
250 mL = 0.250 L
0.85557 mol/0.250 L = 3.422 M
Radio waves, ultraviolet
Radio waves
This is the dumbell shaped orbital type
(l value or letter designation will suffice
p (l=1)
This class of organic compounds is characterized by at least 1 hydroxyl group being attached to a carbon atom of an alkyl group
alcohol
The phase state of this substance:
AgBr
solid
Silver bromide is insoluble
The concentration of ammonium ions if 12 grams of ammonium phosphate ionizes in 50 mL of water
Answer to 3 decimal places
(NH4)3PO4 (aq) --> 3NH4+ (aq) + PO43- (aq)
12 grams (NH4)3PO4 = 0.080488 moles (NH4)3PO4
3 moles NH4 per 1 mole (NH4)3PO4
3 x 0.080488 = 0.241 moles NH4
50 mL = 0.05 L
0.241/0.05 = 4.829 M NH4
This is the value for frequency in hertz given a photon has a wavelength of 800 nanometers
Answer with scientific notation and 2 decimal places
c = λ v
v = c/λ
800 nanometers = 800 x 10-9 meters
v = (3 x 108 m/s) / (800 x 10-9 meters)
v = 3.75 x 1014 Hz
This formula gives the total number of orbitals in a shell with principle quantum number n
n2
e.g. n=4
4s, 4p, 4d, 4f
0; 1,0,-1; 2,1,0,-1,-2; 3,2,1,0,-1,-2,3
16 total orbitals
The IUPAC name for the following compound:
1,3-diethyl-2-methylcyclopentane
The products (with phase states) of a reaction between these 2 substances:
CaS (aq) + Mg(NO3)2 (aq) --> ???
Substitution reaction (double swap), products are:
MgS (s) and Ca(NO3)2 (aq)
The full, balanced equation, with phase states, for the following reaction:
Barium hydroxide solution reacts with hydrochloric acid to form barium chloride solution and water.
Ba(OH)2(aq) + 2HCl(aq) --> BaCl2(aq) + 2H2O(l)
This is the value of the energy of a photon that has a frequency of 1.8 x 104 Hz
Answer in scientific notation, unit in joules, with 2 decimal places
E=hv
E=(6.626x10-34 J*s)(1.8x104 Hz)
E = 1.19 x 10-29 J
These are EVERY possible value for ml given l is equal to 3
3,2,1,0,-1,-2,-3
The name for the following reaction mechanism:
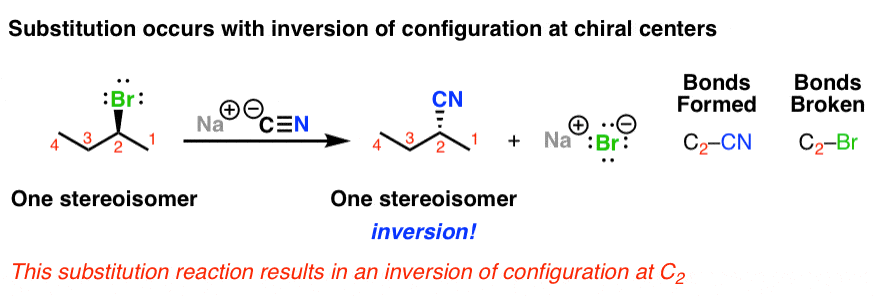
SN2
The products (with phase states) of a reaction between these 2 substances:
AlCl3 (aq) + Na2SO4 (aq) --> ???
Al2(SO4)3 (aq)
NaCl (aq)
300 mL of 3.14 M lead (II) nitrate solution reacts with 550 mL of 2.71 M sodium iodide solution to create 343.6 grams of lead (II) iodide. What would the concentration be of the remaining excess reagent if it was placed in 674 mL of water?
Answer with 4 decimal places
Pb(NO3)2 (aq) + 2NaI (aq) --> PbI2 (s) + 2NaNO3 (aq)
0.3 L Pb(NO3)2 x (3.14 mol Pb(NO3)2)/(1 L PbNO3) x (1 mol PbI2)/(1 mol Pb(NO3)2) = 0.942 mol PbI2
0.55 L NaI x (2.71 mol NaI)/(1 L NaI) x (1 mol PbI2)/(2 mol NaI) = 0.74525 mol PbI2
This means NaI is the limiting reagent
0.942 - 0.74525 = 0.19675 mol Pb(NO3)2
0.19675 mol Pb(NO3)2 / 0.674 L = 0.2919 M Pb(NO3)2
This is the wavelength of a photon in this beam of light given this criteria:
A beam of light is emitted containing 10 joules of energy and a mole of photons.
Answer in scientific notation with meters with 3 decimal places (plus 500 points if you get the type of light it is [e.g. x-ray or ultraviolet], no penalty if you get the light type wrong, only penalized for getting wavelength wrong)
10 Joules/6.022x1023 photons = 1.66057788 x 10-23 Joules per photon
E=hv
c = λ v
E=hc/λ
λ=hc/E
λ=(6.626x10-34J*s)(3x108 m/s)/(1.66057788x10-23 J)
λ = 1.197x10-2 meters (microwave region)
Is this a possible set of quantum numbers for an electron (answer true or false)
n = 4
l = 3
ml = -4
ms = +1/2
false
l = 3
ml can equal 3,2,1,0,-1,-2, or -3, but NOT -4