What is the largest atom on the periodic table?
Francium (Fr)
What types of atoms make up a covalent bond?
Two nonmetals
What is the first step in drawing a Lewis structure?
Count valence electrons!!
What shape has bond angles of 109.5o?
Tetrahedral
What is effective nuclear charge?
The attractive pull that the positively charged nucleus has on the orbiting electrons.
With respects to energy, what happens when a bond breaks?
Input of energy is required break a bond (E>0)
What atoms can exceed the octet rule? Why?
Atoms in n=3 and beyond. These atoms have more energy levels that extra electrons can move into.
What shape has 6 atoms surrounding a central atom?
Octahedral
(Bonus points if you can explain why it's called an OCTAhedral if it has 6 outter atoms, not 8)
Which is larger, Li or Li+? Why?
Li is larger because Li+ has more protons in the nucleus, which pulls the orbiting electrons closer
Order the following from longest to shortest bond length: single bond, double bond, triple bond
single bond > double bond > triple bond
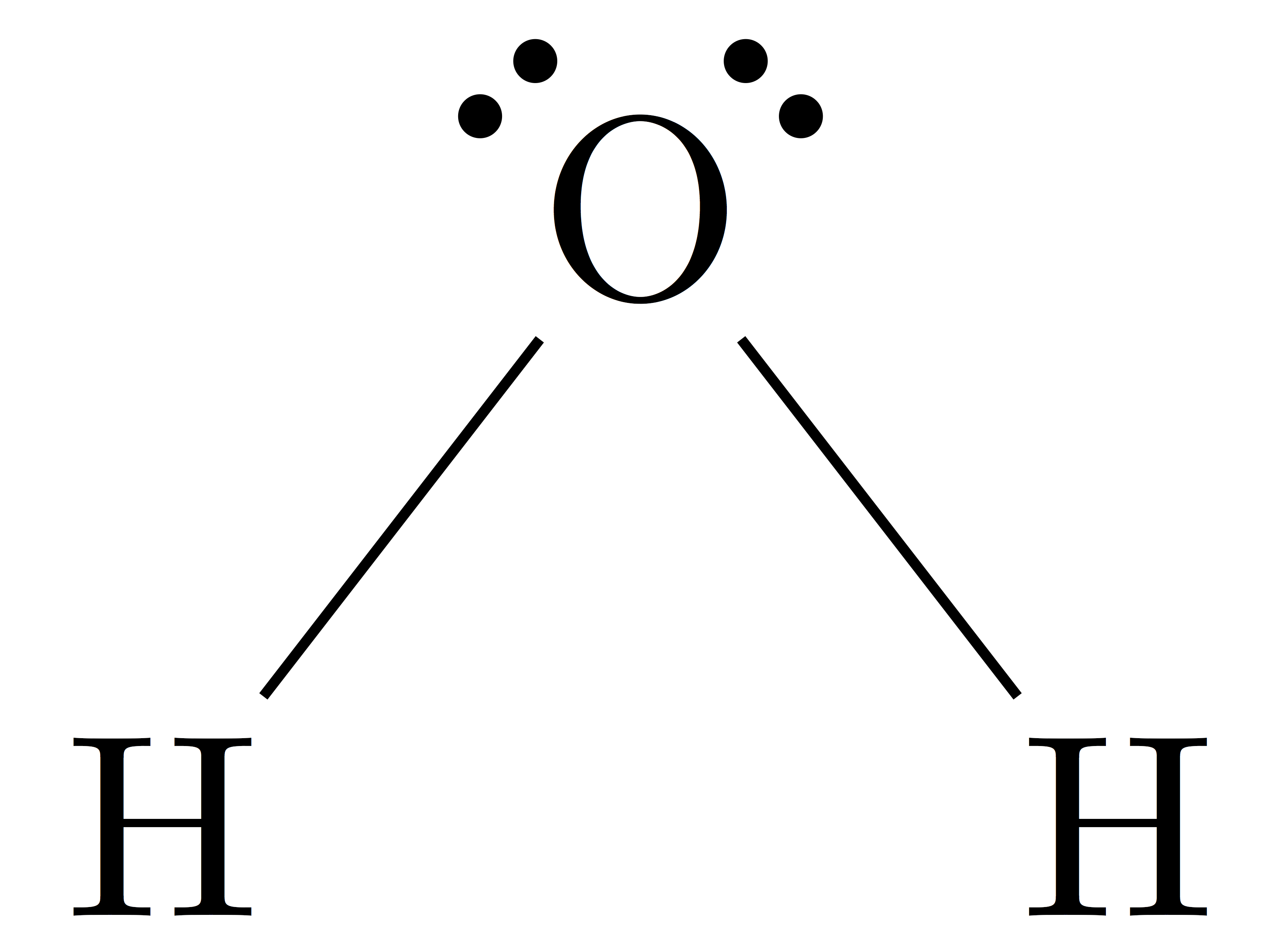
Draw the Lewis structure of water
What is the molecular geometry of CO2?
Linear
Arrange the following in order of decreasing electron affinity: C, Ne, Na, Si
C > Si > Na > Ne
(Ne is the lowest because it is a noble gas, so it has a stable octet and does not want any more electrons)
Which bond is least polar? Why?
O-C
O-Cl
O-H
Cl-H
O-Cl because the electronegativity difference between the two atoms is smallest
What is the formal charge on the central atom of NO3-? (might need to draw it)
-1

What is the molecular geometry of PH3? What is its electron geometry?
Molecular: Trigonal pyramidal
Electron: Tetrahedral

Electronegativity is the power of an atom in a molecule to attract electrons to itself. It increases as you go up a column because shielding of the electron orbitals around the nucleus decreases. It increases as you go to the right on a row because more protons have a stronger attractive force to electrons, and atoms get closer to reaching a stable, noble gas configuration.
How many sigma bonds and pi bonds are in the molecule?

18 sigma bonds
5 pi bonds
Draw the Lewis structure of PO43-. How many possible resonance structures are there?
4 possible resonance structures

Determine the shape and bond angles of SF4
Seesaw; Bond angles of <90o and <120o
![]()