The equation for radioactive decay
What is parent nucleus --> daughter nucleus + decay particle
The effect of lone pairs on bond angle
what is the lone pair will decrease (or increase) the ideal angle
The hybridization of the two carbon atoms in acetic acid?
what is H3C, sp3; C(O)OH, sp2
The rank of the intermolecular forces from strongest to weakest
What is hydrogen bonding > dipole-dipole > London dispersion forces
The difference between crystalline and amorphous solids
what is crystalline solids have repeating patterns while amorphous solids are characterized by their lack of order
The completed equation for the following beta emission: 13153I --> ? + 0-1e
what is 13153I --> 13154Xe + 0-1e
The relationship between electronegativity and bond polarity
what is electronegativity determines polarity in a molecule
The bond angle between the two H atoms:
What is less than 109.5 degrees
The phase change from a solid directly to a gas
what is sublimation
The hybridization of the central atom of the following molecule: Cl2SO (S is the central atom)
what is sp3
The definition for degenerate
what is having the same energy
The bond with the shortest length in the following Lewis Structure 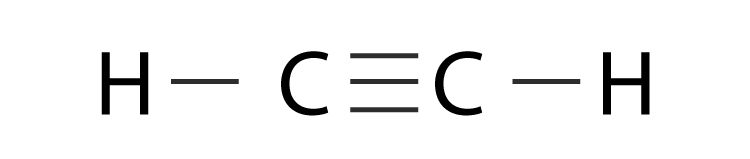
The bond order of N2
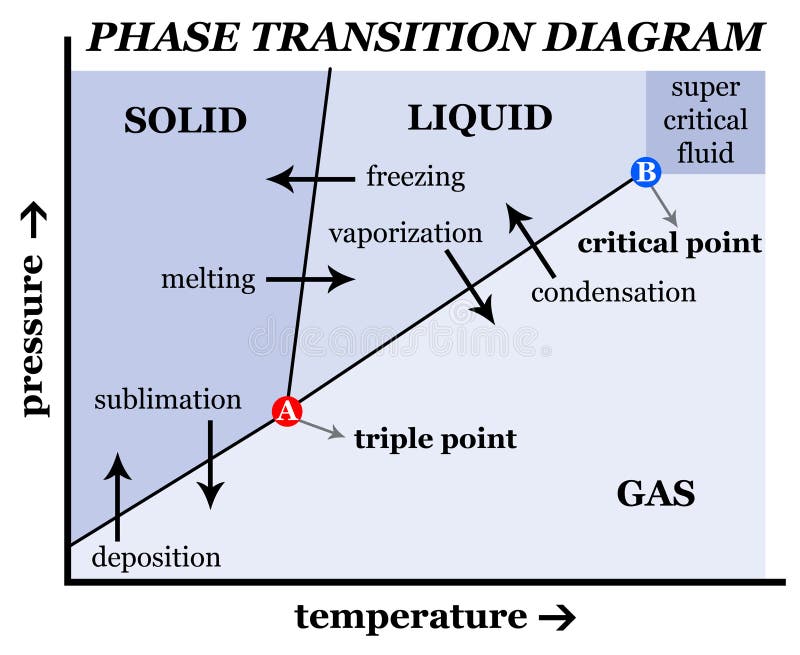
The labels for the following blank phase diagram
The polarity (nonpolar or polar) of a molecule that has lone pairs is likely to be...
what is polar
The competed equation for the following positron emission 116C --> ? + 01e
what is 116C --> 115B + 01e
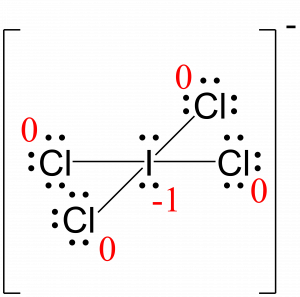
The Lewis Structure for ICl4-
The number of pi bonds and sigma bonds for this illustration:
What is 9 sigma bonds and 2 pi bonds
The aspects of a molecule that can raise the amount of dispersion forces to overcome
The name of the following compounds:
(a) CsCl
(b) BaO
(c) K2S
(d) BeCl2
(e) HBr
(f) AlF3
what is (a) cesium chloride; (b) barium oxide; (c) potassium sulfide; (d) beryllium chloride; (e) hydrogen bromide; (f) aluminum fluoride
The symbol for the Alpha decay particle
what is 42He
The electron domain geometry and molecular geometry for ClF3
What is trigonal bipyramidal (electron domain geometry) and T-shaped (molecular geometry)
The charge that would be needed on F2 to generate an ion with a bond order of 2
what is 2+
The arrangement of the following molecules from lowest boiling point to highest boiling point: HCl, H2O, SiH4
what is SiH4 < HCl < H2O
The bond order of each of the following: F2, F2+, F2− and identify the strongest bond of the three
F2 bond order = 1, F2+ bond order = 1.5, F2− bond order = 0.5, strongest bond is F2+