What state of matter does the following image depict?

Gas
How many degrees Celsius (°C) is 400°K?
127 °C
What is an exothermic reaction?
energy released
Is this an element, compound or mixture?
.jpg)
What is the molar mass of Zn(OH)₂ ?
99.38 g/mol
What type of bond would occur between Na and S?
ionic
51.6 grams of sugar occupies a volume of 3 mL. What is the density of the sugar in g/mL?
17.2 g/mL
Convert 738 mmHg to atmospheres (atm).
0.97 atm
If water, what temperature would you find at part D?
100
Which of the following is not a correct atomic symbol? Cu, C, cl, Co
cl
What is the empirical formula for a compound that has 2.00 moles of A and 4 moles of B?
AB2
What is the name for PCl5 ?
phosphorus pentachloride
How many sig figs are in 0.00500
3
The pressure in a tire is 105 psi at 25˚C in Fresno. You take the bicycle up to Huntington, where the temperature is – 5˚C. What is the pressure in the tire?
(V and n = constant)
94.4 psi
What phase earns the most bars on an LOL diagram?
gas
Would filtration, distillation, or electrolysis work best for separating a water and salt solution?
distillation
How many moles are in 36 grams of water?
2
What is the formula for Copper (II) Nitrite?
Convert 1.00 g to Kg
0.001 Kg
What would be the new pressure if 250 cm3 of gas at standard pressure is compressed to a volume of 150 cm3 ? (n and T = constant)
1.7 atm
Calculate the amount of heat needed to raise the temperature of 150.0 g of water from 15.0°C to 40.0°C
+15675 J
Separating water into Hydrogen gas and Oxygen gas is a chemical change that we can do using what type of process?
electrolysis
A lab experiment calls for 4.5 moles of ZnCl2. How many grams of ZnCl2 will you use?
613
How many ions are created when Na3PO4 dissociates?
4
Convert 1,230,000 to scientific notation
1.23 e 6
If number of particles and temperature are held constant, what happens to the volume of a gas as pressure increases?
Decreases
Inverse relationship.
What is the specific heat of 100g of liquid when 1000J of energy is gained and heated 20˚C to 80˚ C
0.167 J/g ˚C
Is C an element, compound or mixture?
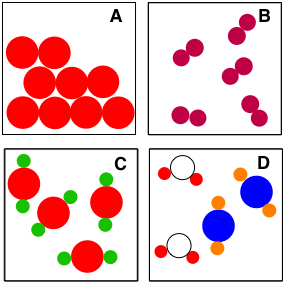
Compound
What is the percent composition of ducks in the picture?
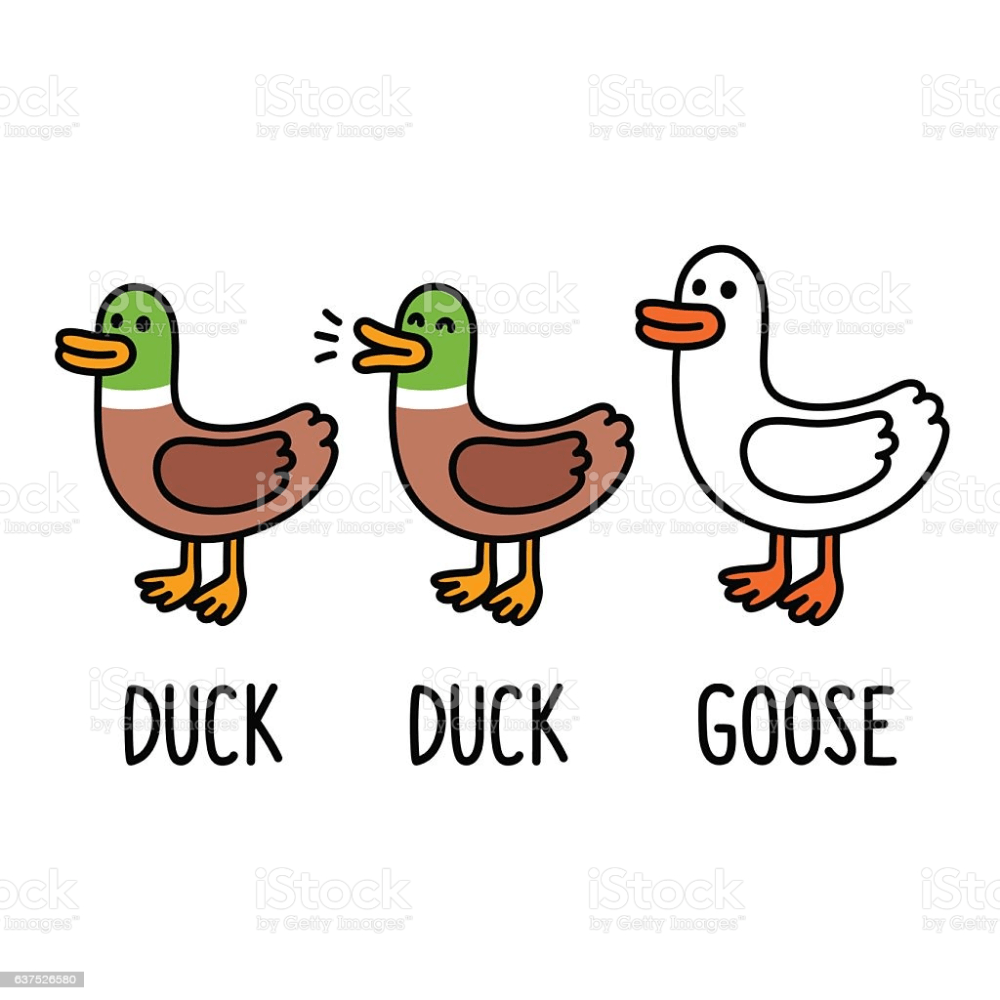
66%
What is the name for KC2H3O2 ?
potassium acetate