What are the 7 diatomic elements?
H, N, O, F, Cl, Br, I
What do the coefficients in the following reaction represent?
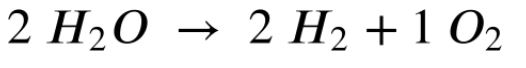
Moles or Molecules
What are the typical units for Temperature in PV=nRT?
K
What are the two particles that have the same mass?
n and p
What is the electron configuration for Boron?
[He] 2s2 2p1
1s2 2s2 2p1
Very weak attractive forces because of the momentary unequal distribution of electrons around an atom due to continuous movement of electrons in their orbitals. Sometimes they happen to be clustered.
LDF
What always occurs at chemical equilibrium?
The forward reaction and reverse reaction occur at the same rate or speed.
Something with a pH of 9 would be classified as what?
Base
What is the ratio of the coefficients?
__CH4 + __O2 → __CO2 + __H2O
1:2:1:2
How many moles of potassium would be required for this reaction?
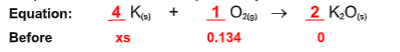
0.536
As temperature goes up, with everything held constant, what happens to pressure?
Increases
Rubidium is a soft, silvery‐white metal that has two common isotopes, Rb-85 and Rb-87. If the abundance of Rb-85 is 72.2% and the abundance of Rb-87 is 27.8%, what is the average atomic mass of rubidium?
85.556 amu's
Neon, a noble gas, has a filled valence level. Which of the following has an identical electron configuration?
A. Cl-
B. Mg+
C. O2-
D. N2-
E. Ga3+
C
Salt water is a good example of what IMF?
I-D
What side of the equation is "HEAT" on for an exothermic reaction?
Products
The pH scale ranges from 0 to what?
14
What is the ratio of the coefficients?
__AgNO3 + __K2SO4 → __Ag2SO4 + __KNO3
2:1:1:2
11.34 grams of Carbon Dioxide is recovered, what is the percent yield?
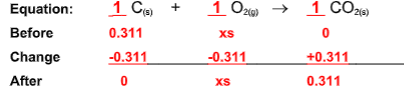
82.8%
At STP, what is the volume of one mole of CO2 gas?
22.4 L
Naturally occurring chlorine that is put in pools is 75.53 percent Cl-35 (mass = 34.969 amu) and 24.47 percent Cl-37 (mass = 36.966 amu). Calculate the average atomic mass.
35.46 amu
Which of the following elements has the greatest first ionization energy?
A. Lithium
B. Calcium
C. Chlorine
D. Silicon
C
Methane is made of a 1 carbon chain and pentane is made of a 5 carbon chain, which has the higher boiling point?
Pentane
K < 1 favors what at equilibrium?
Reactants
Is Calcium Hydroxide a strong or weak base?
Strong
What type of reaction is depicted below?
_1_N2 +_2_H2O → _1_NH4NO2
synthesis
What is the excess reactant?
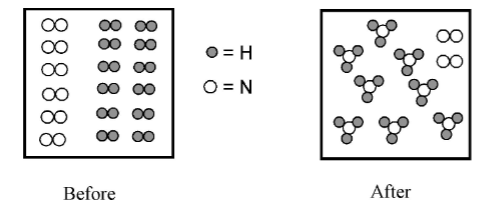
Nitrogen
If you collected 45 mL of H2 gas at about 25 ⁰C and 0.97 atm, what would the volume of hydrogen gas be if changed to STP?
39.98 mL
If an element has 9 protons, 10 neutrons and has a -1 charge, how many electrons does it have?
10
The atom with the largest atomic radius in Group 18 is?
Rn
What atoms need to be involved for Hydrogen Bonding to occur?
FON
Fe3+(yellow) + SCN-1(colorless) <--> [FeSCN]2+(dark red)
Write the equilibrium expression.
What is a neutralization reaction?
produces water and a salt/ionic compound
What type of reaction is depicted below?
__CH3OH + __O2 → __CO2 + __H2O
combustion
How many grams of excess reactant remain?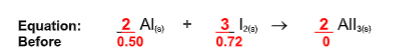
0.54 g
You find experimentally that 15.0 kJ of energy is released when 0.002 moles of paraffin wax burns. How much energy would be released if 1 mole of paraffin wax burned?
7,500 kJ / mole
What are the x and y axis labels on a mass spectrum graph?
x - isotope mass
y - % abundance
The element with the lowest electronegativity in the Alkaline Earth Metals Family is?
Radium
The C-O bond is considered polar covalent; however, CO is polar and CO2 is nonpolar? Why?
CO is asymmetrical whereas CO2 is symmetrical. This means that the dipole moment arrows in CO2 cancel out and the molecule is nonpolar overall.
2 HCl(aq) + Mg(s) <--> MgCl2(aq) + H2(g) + heat
What happens when temperature is decreased?
Shift to products
What is the pH of something that has a [H3O+] of 0.10 M?
1