The total number of valence electrons in Nitric Acid(HNO3)
What is 24?
The molar mass of Carbonic Acid
What is 62.03g/mol?
The number of oxygens in Phosphorous acid
What is 4 oxygens?
The molecular geometry of CO2
What is linear?
The number of carbon atoms in butane.
What is 4?
The total number of valence electrons in Formic Acid(HCOOH)
What is 18?
The number of oxygen atoms in 60.0 grams of carbon dioxide
What is 1.64 * 10^24 O atoms
The chemical formula for Magnesium nitrate
What is Mg(NO3)2
The electronic geometry of Oxygen in water
What is tetrahedral?
The functional group with the highest priority.
What is carboxylic acid?
Number of double bonds in Carbonate Ion
What is 1?
The number of GRAMS of Oxygen atoms in 6 moles of acetic acid
What is 192g of O?
The molecule name of ClO2^-
What is Chlorite?
The hybridization of Nitrogen in Ammonium
What is sp^3?
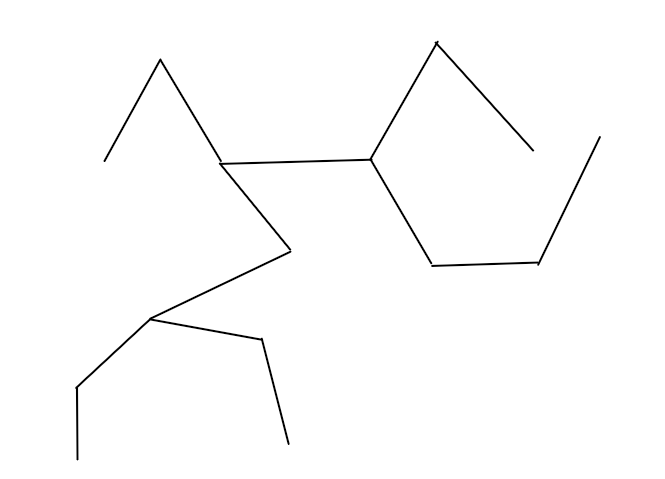
What is 3,5,6-triethylnonane?
The number of resonance structures for the nitrate ion that satisfy the octet rule
What is 3?
Consider the reaction: 2NaBr(aq) +Cl2(g) -> 2NaCl(s) +Br2(g)
If 45 grams of sodium bromide is reacted with 25 grams of chlorine gas, how many grams of sodium chloride can we expect to form? What is the LR?
About 25.5 grams of sodium chloride (NaCl). LR is sodium bromide (NaBr).
The chemical formula for sulfurous acid
What is H2SO3?
The hybridization of the Carbon atom in carbon monoxide
What is sp hybridized?
What functional groups does this structure contain?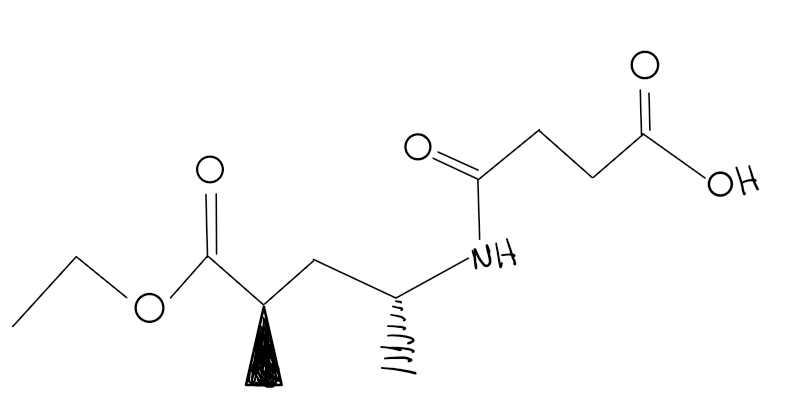
Ester, Amide, and carboxylic acid
The formal charge of the C atom in the Formate ion(HCO2^-)
What is 0?
If 103 grams of potassium sulfite are dissolved in 657mL of aqueous solution, the potassium ion concentration is approximately...(Hint: Use Molarity formula)
1.98M
The molecule name of CH3COO^-
What is acetate?
Acetone is the common name for 2-propanone. What is the hybridization and electronic geometry of the center carbon atom in the molecule?
sp^2 hybridized and trigonal planar
What is the name of this organic structure?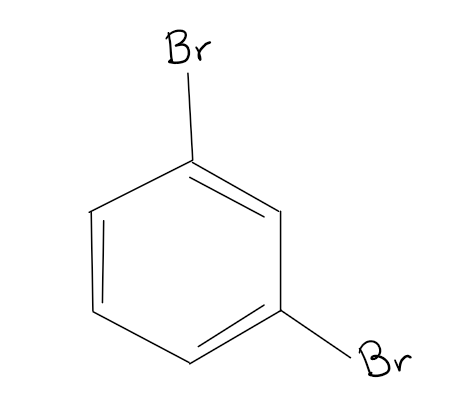
1,3-dibromobenzene or meta-Dibromobenzene