How many electrons can the second and third shell fill within an atom?
8
Where are the electrons located in an atom?
Electron cloud, energy shells, orbitals
What is a group?
A column on the periodic table
What is a period?
A row on the periodic table
In an ionic bond, atoms ____________ valence electrons. However, in a covalent bond, atoms ________ valence electrons.
transfer, share
Which group on the periodic table are stable elements/atoms and do not want to react with other atoms?
Which two subatomic particles make up the nucleus?
Protons and neutrons
State the atomic number, mass and number of neutrons for the following isotope. 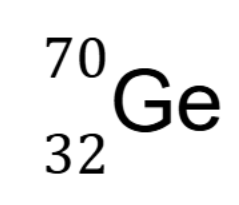
Atomic number: 32
Mass: 70
# of neutrons: 38
An element’s period number tells us the ____________________________ (# of valence electrons/ # of orbitals or shells) in an atom within that period.
# of orbitals/ shells
An element’s group number tells us the ____________________________ (# of valence electrons/ # of orbitals or shells) in an atom within that group.
# of valence electrons
An ionic bond is a bond that involves what two types of elements/atoms? (metal/nonmetal/metalloid)
Metal and nonmetal
A covalent bond is a bond that involves what two types of elements? (metal/nonmetal/metalloid)
2 nonmetals
Write the symbol of the isotope using the given information. Use your study guide for reference. DO NOT USE THE PERIODIC TABLE FOR MASS.
An isotope with 14 protons and 16 neutrons.
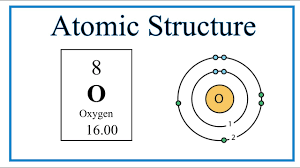
Draw a Bohr model of oxygen.
What group on the periodic table are highly reactive nonmetals?
The Halogens (group 17)
What group on the periodic table are highly reactive metals?
Alkaline Metals
Is the following ionic bond a single, double or triple bond?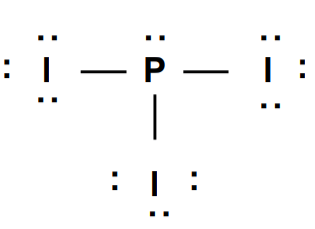
single (3 single bonds)
Write the formula for disilicon hexabromide
S2Br6
Find the missing information for the unknown element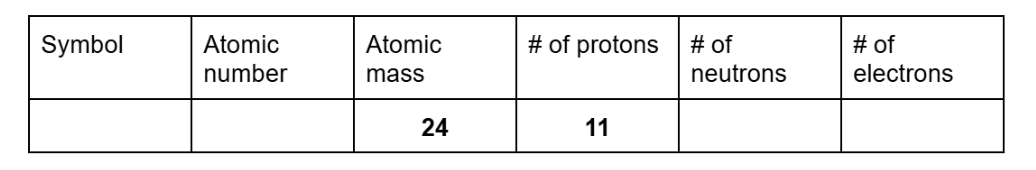
Symbol: Na
Atomic number and electrons: 11
Neutrons: 13
What is an isotope?
Atoms of the same element with a different number of neutrons and different atomic mass
State the group number and # of valence electrons for the following group:
Alkaline Earth metals
2 and 2
State the group number(s) of the following group:
Transition metals
3-12
The following picture shows the transfer of electrons within the ionic bond Magnesium Iodide. State the charges for each cation and anion within the bond. 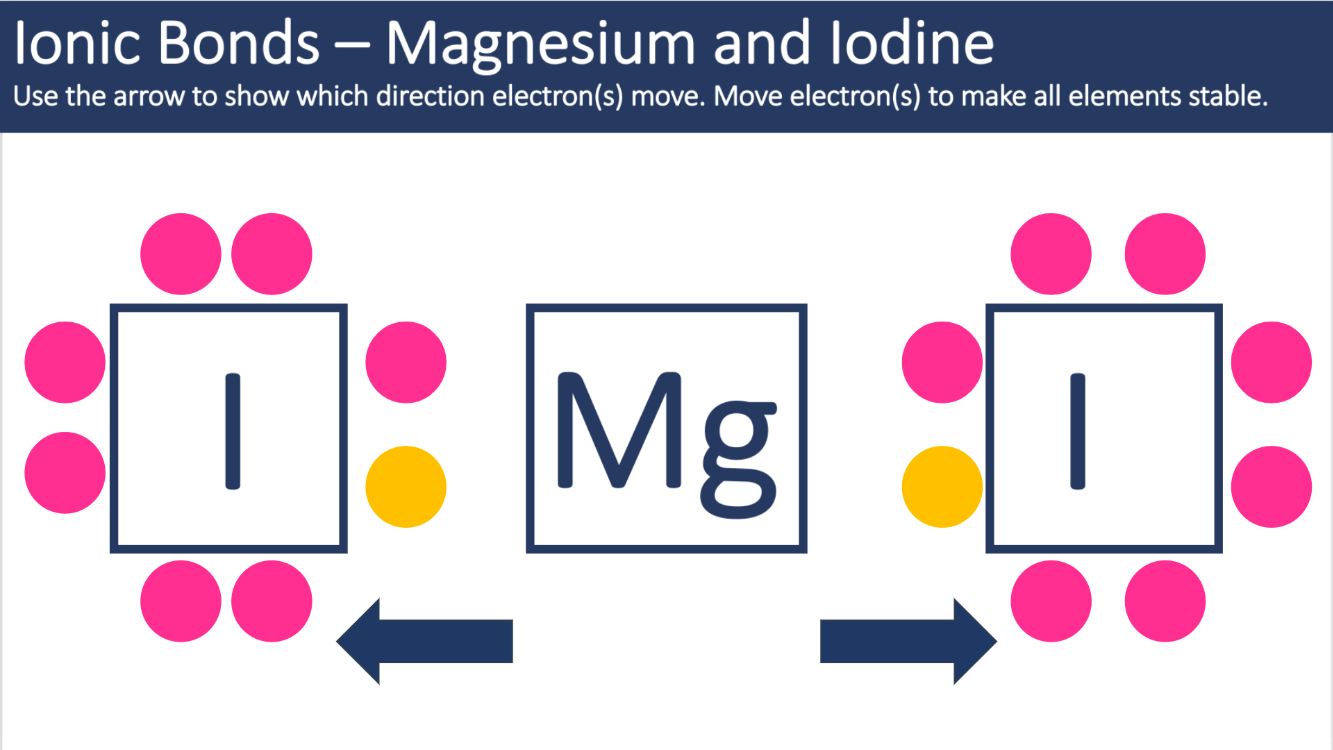
Mg2+ I- I-
Write the name of the following covalent compound.
N2O5
What is a metalloid?
Name 3 examples of metalloids.
Boron, silicon, germanium, arsenic, antimony, and tellurium
Show the transfer of electrons for the following ionic bond then name the ionic bond:
LiF
Lithium Fluoride
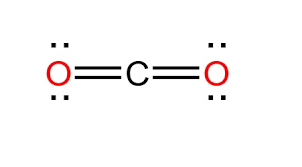
Create a line structure for the following covalent bond:
CO2