How many significant figures are in the following measurement?
4.0904 m
5
Which scientist created the plum pudding model of the atom?
J. J. Thompson
State the full electron configuration of iron (Fe)
1s22s22p63s23p64s23d6
What entire group of elements was left out of Mendeleev's original periodic table? Why?
Name one difference between ionic and covalent bonds
answers may vary
Which of these measurements is the most precise?
a) 2.04 m
b) 3.1 m
c) 1.267 m
c - it has the highest number of sig figs
Which scientist created the planetary model of the atom?
Niels Bohr
State the condensed electron configuration of titanium (Ti)
[Ar]4s23d2
An atom's attraction to the electrons in a bond
What is the octet rule?
Elements tend to bond in such a way that each atom has eight electrons in its valence shell, giving it the same electronic configuration as a noble gas.
What is the difference between accuracy and precision?
Accuracy is determined by how close a measurement is to reality, while precision is determined by the number of sig figs in a measurement
What is the difference between a compound and a mixture?
A compound consists of two or more elements that are bonded chemically, while a mixture consists of two or more substances that are mixed together but not bonded.
How many electrons can a p subshell hold?
6
How can you determine if a covalently bonded molecule is polar or nonpolar?
Determine the electronegativity values of the elements involved and take the difference of them. If the difference is below 0.5, the bond is nonpolar; if the difference is between 0.5 and 1.7, the bond is polar.
Write the chemical formula for Tin (IV) sulfide
SnS2
Convert 498 nm to m
4.98 x 10-7 m
Calculate the molar mass of sucrose, C12H22O11
342.30 g/mol
A lab sample consists of 4.58% H, 40.92% C, and 54.51% O. Determine the empirical formula for this substance
C3H4O3
Why is a bent molecular geometry bent?
The lone pairs of electrons pull away from the central atom more strongly than the bonded pairs. This causes the angles between the bonded pairs to decrease, resulting in a bent shape.
Name the following compound: MnSO4
Convert 87.21 L/s to mL/min
5.233 x 106 mL/min
28.32 moles
A compound has an empirical formula of CH2O and a molar mass of 120.12 g/mol. Determine the molecular formula for this compound.
C4H8O4
Determine the molecular geometry of ammonia, NH3
trigonal pyramidal
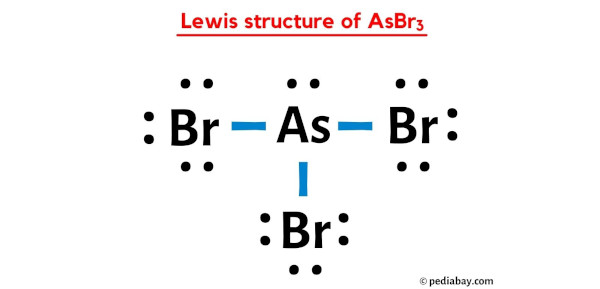
Draw the Lewis Structure for Arsenic tribromide, AsBr3