PV=nRT
What is 50 degrees Celsius in Kelvin?
323 K
How many pi and sigma bonds are in a triple bond?
one sigma and two pi bonds
Who discovered the atomic theory?
Dalton
What do you change when balancing a chemical equation?
Coefficients
What is Dalton's Law of Partial Pressure?
Pt=P1+P2+P3...
A gas occupies 4.98 L at 2.6 atm of pressure. What volume does it occupy at 1.8 atm pressure?
7.2 L
how many pi and sigma bonds are in a single bond
1 sigma and 0 pi
Who discovered the Electron?
JJ Thomson
What is in a covalent bond and an ionic bond?
Covalent=nonmetal + nonmetal
Ionic= metal +nonmetal
What is Charles' Law?
V1/T1=V2/T2
What is the molarity of a 0.5 L sample of a solution that contains 60.0 g of NaOH?
3.0 M
What is the electron domain geometry of an atom with 4 electron domains?
Tetrahedral
What experiment did Millikan conduct that determined the charge on the electron?
Oil drop experiment
What are all the symbols of quantum numbers?
n, l, ml, ms
What is Boyle's Law?
P1V1=P2V2
Suppose a hydrochloric acid solutions contains 36 g of HCl and 64 g of H2O, What is the mole fraction of HCl?
0.22
What is the Molecular geometry of an atom with 2 bonding domains and 0 nonbonding domains?
Linear
What are the three types of radiation that Rutherford discovered?
alpha (positive charge), beta (negative charge), and gamma rays (uncharged)
How many valence electrons does the atom Bi (#83) have?
5 valence electrons
What is the equation for Energy?
E=hv=hc/wavelength
What is the molality of a solution made by dissolving 2 moles of NaOH in 400 grams of water?
5 mol/kg
What is the molecular geometry of an atom with 4 bonding domains and 1 nonbonding domain?
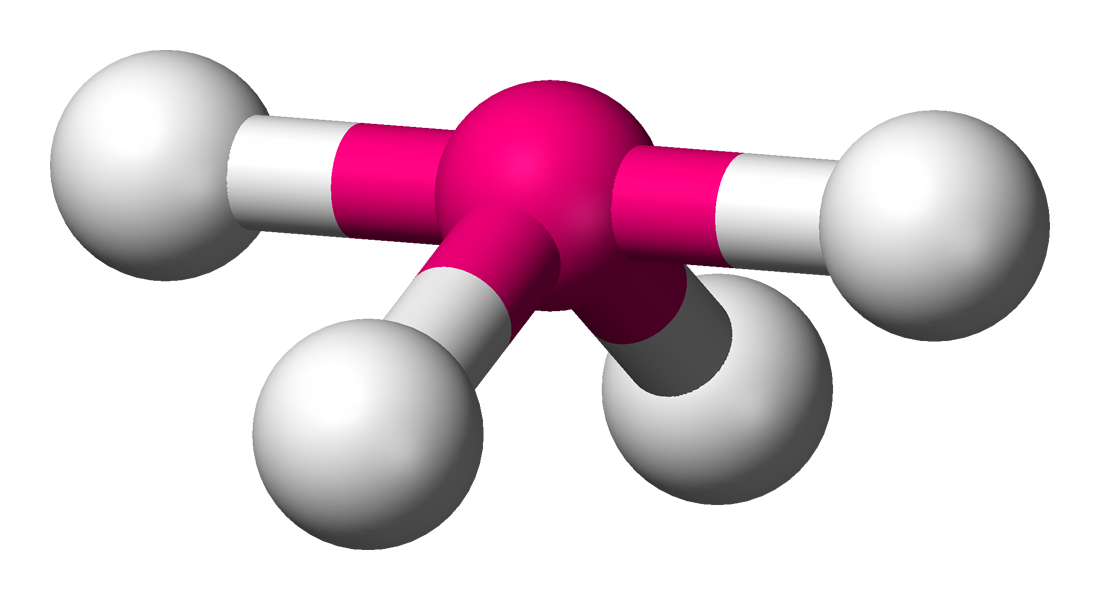
Seesaw
Who's number is 6.02x1023?
Avogadro's number
What is the VSEPR theory?
The best arrangement of a given number of electron domains is the one that minimizes the repulsions among them.
(predicts shapes of molecules)