One resonance structure for thiocyanate ion is drawn below. What is the formal charge on each atom?
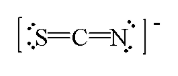
a. S = 0, C = +2, N = -3
b. S = 0, C = -1, N = 0
c. S = 0, C = 0, N = -1
d. S = -2, C = +4, N = -3
e. S = -1, C = 0, N = 0
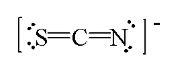
c. S = 0, C = 0, N = -1
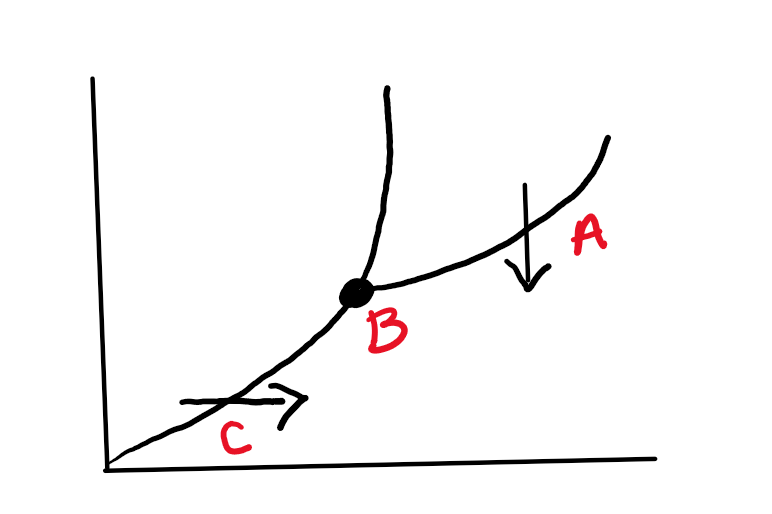
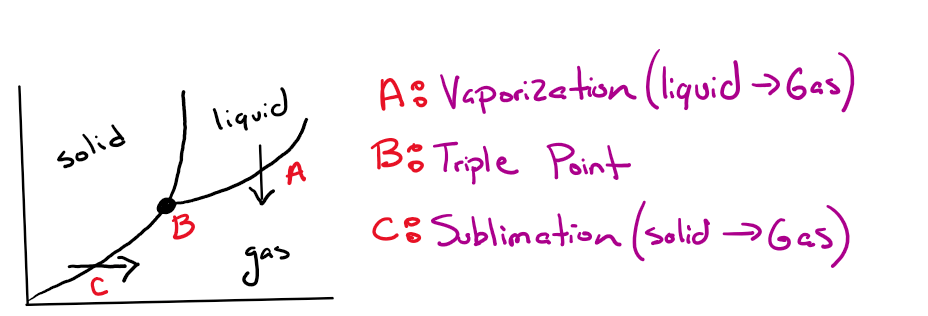
What do the point and arrows demonstrate respectively?
% by mass equation
(mass solute (g)/ total mass of solution (g)) *100
Label the energy diagram below as exothermic or endothermic
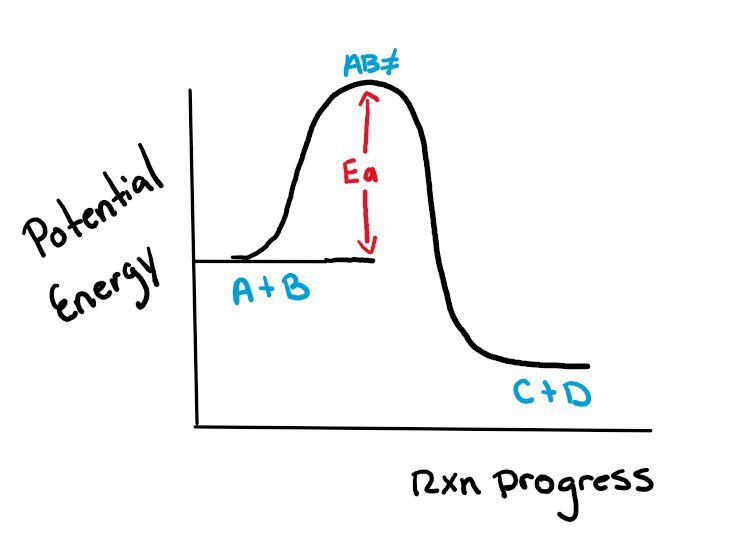
Exothermic
According to the Brønsted-Lowry definition, a base
a. increases the H3O+ concentration in an aqueous solution.
b. increases the OH- concentration in an aqueous solution.
c. is a proton acceptor.
d. is a proton donor.
e. is an electron-pair donor.
c. is a proton acceptor.
A saturated solution means the reaction is at equilibrium
A) True
B)False
A) True
Which of these species has the highest entropy (S°) at 25°C?
A) CH3OH(l) B) CO(g) C) MgCO3(s) D) H2O(l) E) Ni(s)
B) CO(g)
Who would win: 100 men Vs. 1 gorilla
Chat says 100 men
Which of the following hydrocarbons has two π bonds?
a.C4H6
b.C7H14
c.C3H6
d.C6H12
e.C8H18
a. C4H6
What intermolecular force or bond is primarily responsible for the solubility of chlorine (Cl2) in water?
a. dipole/dipole force
b. hydrogen bonding
c. dipole/induced dipole force
d. ion-dipole force
e. ion-induced dipole force
c. dipole/induced dipole force
Molality Equation
moles solute/ kg of solvent
A 0th order reactant cannot influence a reaction based on its concentration
A) True
B) False
A) True
What are the strong acids?
HI, HCl, HBr, HNO3, HClO3, HClO4, H2SO4
Sodium Nitrate is insoluble in water
A) True
B)False
B) False
Determine the oxidation number of Sulfur in H2SO4
S=+6
Which U.S. document begins with “We the People”?
The Constitution
What is the hybridization of the sulfur atom in SO2?
a) sp3d2
b)sp
c) sp2
d) sp3d
c) sp2
Which of the following hydrocarbons can have cis and trans isomers?
a. propene
b. 1-butene
c. 2-ethylbutane
d. 2-pentyne
e. 3-hexene
e. 3-hexene
Which of the following changes are likely to increase the rate of a chemical reaction?
1. Adding a catalyst
2. Increasing the temperature of the reactants
3. Increasing the concentrations of the reactants
a. 1 only
b. 2 only
c. 3 only
d. 1 and 3
e. 1, 2, and 3
e. 1, 2, and 3
A gaseous mixture of NO2 and N2O4 is in equilibrium. If the concentration of N2O4 is 7.1 × 10-4 M, what is the concentration of NO2?
2 NO2(g) ⇋ N2O4(g) Kc = 170
a. 1.7 × 10-11 M b. 4.2 × 10-6 M
c. 2.0 × 10-3 M d. 4.9 × 102 M
e. 2.4 × 105 M
c. 2.0 × 10-3 M
Identify the acid and conjugate base of the following reaction
HCO3-+ HPO42- -> H2CO3 + PO43-
Acid: HPO42-
Conj. Base: PO43-
A titration of an acid and base to the equivalence point results in a noticeably acidic solution. It is likely this titration involves
A) a strong acid and a weak base.
B) a weak acid and a strong base.
C) a weak acid and a weak base (where Ka equals Kb).
D) a strong acid and a strong base.
A) a strong acid and a weak base.
A certain electrochemical cell has for its cell reaction:
Zn + HgO → ZnO + Hg
Which is the half-reaction occurring at the anode?
A) HgO + 2e- → Hg + O2– C) Zn → Zn2+ + 2e-
B) Zn2+ + 2e- → Zn D) ZnO + 2e- → Zn
C) Zn → Zn2+ + 2e-
In Breaking Bad, what is Walter White’s street name?
Heisenberg
Which of the following is a correct Lewis structure for N2O?
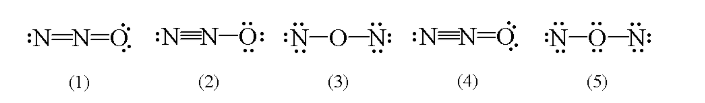
(2)
An unsaturated hydrocarbon is
a. a compound in which all carbon atoms have four single bonds.
b. a cycloalkane with five or more carbons.
c. a hydrocarbon that is a gas at room temperature.
d. a compound in which one or more carbon atoms have double or triple bonds.
e. a hydrocarbon that contains oxygen.
d. a compound in which one or more carbon atoms have double or triple bonds.
How are the exponents in a rate law determined?
1. They are equal to the concentrations of the reactants.
2. They are equal to the coefficients in the balanced chemical equation.
3. They are determined by experimentation.
a. 1 only
b. 2 only
c. 3 only
d. 1 and 2
e. 2 and 3
c. 3 only
What is the overall order of the reaction below
2 NO(g) + 2 H2(g) → N2(g) + 2 H2O(g)
if it proceeds via the following rate law?
rate= k[NO]2[H2]
a. zero-order
b. first-order
c. second-order
d. third-order
e. fourth-order
d. third-order
Which one of these salts will form a basic solution on dissolving in water?
A) KCl B) KI C) NaNO2 D) NH4NO3 E) KBr
C) NaNO2
Find the normality of 15M of H2SO3
30 N
N= nM
Aluminum forms a layer of aluminum oxide when exposed to air which protects the bulk metal from further corrosion.
4Al(s) + 3O2(g) → 2Al2O3(s)
Using the thermodynamic data provided below, calculate ΔS° for this reaction.
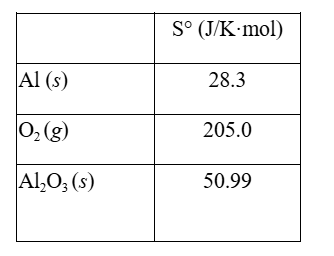
A) 182.3 J/K·mol D) –626.2 J/K·mol
B) 131.5 J/K·mol E) –802.9 J/K·mol
C) –182.3 J/K·mol
D) –626.2 J/K·mol
How many colors are there in a rainbow?
7

How many sigma (σ) bonds and pi (π) bonds are in acetic acid?
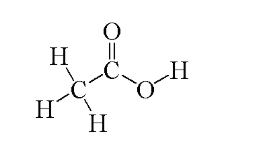
7 sigma and 1 pi bond
What is the balanced chemical equation for the combustion of 2-methyl-1-butene?
a. 2C4H10(g) + 13O2(g) → 8CO2(g) + 10H2O(l)
b. 2 C5H10(g) + 15O2(g) → 10CO2(g) + 10H2O(l)
c. C4H8(g) + 6O2(g) → 4CO2(g) + 4H2O(l)
d. C4H10(g) + 13O2(g) → 5CO2(g) + 6H2O(l)
e. C5H12(g) + 9O2(g) → 6CO2(g) + 6H2O(l)
a. 2C4H10(g)+ 13O2(g) → 8CO2(g) + 10H2O(l)
The Henry's law constant for N2 in water at 37 °C is
8.2 × 10-7 M/mm Hg. What is the equilibrium concentration of N2 in water when the partial pressure of N2 is 634 mm Hg?
a. 1.3 × 10-9 M
b. 5.2 × 10-4 M
c. 1.9 × 10-2 M
d. 1.9 × 103 M
e. 7.7 × 108 M
b. 5.2 *10-4
Henry's Law (C=kP)
The half-life of a first-order decomposition reaction is 2.36 hours. If the initial concentration of reactant is 0.52 M, what is the concentration of reactant after 752 seconds?
a. 0.046 M
b. 0.16 M
c. 0.26 M
d. 0.47 M
e. 0.49 M
e) 0.49 M
*use first order concentration & half-life equations*
What is the H+ ion concentration in a 4.8*10-2 M KOH solution?
A) 4.8* 10-2 M B) 4.8*10-12 M C) 1.0* 10-7 M
D) 2.1* 10-13 M E) 4.8*10-11 M
D) 2.1*10-13
At 1500°C the equilibrium constant for the reaction CO(g) + 2H2(g) ⇋ CH3OH(g) has the value
Kp = 1.4 × 10-7. Calculate ΔG° for this reaction at 1500°C. (R=8.314 J/mol*K)
A) 105 kJ/mol D) –105 kJ/mol
B) 1.07 kJ/mol E) 233 kJ/mol
C) –233 kJ/mol
E) 233 kJ/mol
ΔG°=−RTlnK
Determine the overall cell potential of the following redox reaction
PbCl2(s) + 2 Li(s) → Pb(s) + 2 Li+(aq) + 2 Cl-(aq)
E°cell= 2.92 V
What does URL stand for
Uniform Resource Locator
Calculate the number of chlorine atoms in 441g calcium chloride.
4.79*1024 chlorine atoms in 441g of CaCl2
At its boiling point of -42.1 °C, 14.6 kJ of heat (q) is required to vaporize 33.9 g of propane (C3H8). What is the molar enthalpy of vaporization of propane? (Hint: Heating Curves graph)
a. 2.32 kJ/mol
b. 11.2 kJ/mol
c. 19.0 kJ/mol
d. 97.8 kJ/mol
e. 473 kJ/mol
c. 19.0 kJ/mol
q=nΔHvap
A 9.50 % by mass solution of acetone (C3H6O) in water has a density of 0.9849 g/mL at 20°C. What is the molarity of this solution?
A) 0.621 M
B) 1.61 M
C) 1.66 M
D) 1.71 M
E) 16.9 M
B) 1.61M
Phosgene, COCl2, a poisonous gas, decomposes according to the equation COCl2(g)->CO(g) + Cl2(g). Calculate Kp for this reaction if Kc = 0.083 at 900 degrees C. (R=0.0821 L*atm/mol *K)
A) 0.125
B) 8.0
C) 6.1
D) 0.16
B) 8.0
Kp=Kc(RT)Δ n
What is the H3O+ concentration of an aqueous solution with a pH of 12.17?
a. 6.8 × 10-13 M b. 1.9 × 10-9 M
c. 5.2 × 10-6 M d. 1.5 × 10-2 M e. 1.1 M
a. 6.8 × 10-13 M
10-12.17 =[H]
Calculate the pH of a buffer solution that contains 0.25 M benzoic acid (C6H5COOH) and 0.15M sodium benzoate (C6H5COONa). [Ka = 6.5*10-5 for benzoic acid]
A) 3.97 B) 4.83 C) 4.19 D) 3.40 E) 4.41
A) 3.97
(pH=-log(Ka)+log(conj. base/ acid)
Complete and balance the following redox equation that occurs in acidic solution using the set of smallest whole-number coefficients. What is the sum of all the coefficients in the equation?
PbO2(s) + Cl- → Pb2+ + Cl2(g) (acidic solution)
A) 2 B) 4 C) 5 D) 9 E) 11
E) 11
In George Orwell’s 1984, what is the name of the totalitarian regime’s leader?
Big Brother
Use VSEPR theory to predict the electron-pair geometry and the molecular geometry of iodine trichloride, ICl3.
A) The e- pair geometry is tetrahedral, the molecular geometry is trigonal-planar.
B) The e- pair geometry is trigonal-bipyramidal, the molecular geometry is T-shaped.
C) The e- pair geometry is tetrahedral, the molecular geometry is trigonal-pyramidal.
D) The e- pair geometry is trigonal-planar, the molecular geometry is trigonal-planar.
E) The e- pair geometry is trigonal-bipyramidal, the molecular geometry is trigonal-planar.
B) The e- pair geometry is trigonal-bipyramidal, the molecular geometry is T-shaped.
Hydrogen bonding is present in which of the following molecular solids?
1) Formic Acid
2) Dimethyl Ether
3) Ammonia
4) Pentanol
A) 1, 2, and 4
B) 1, 3, and 4
C) 1, 2, 3, and 4
D) none of them
B) 1, 3, and 4
What is the osmotic pressure of a solution that contains 13.7 g of propyl alcohol (C3H7OH) dissolved in enough water to make 500 mL of solution at 27°C? (R=0.0821 atm/M*K)
A) 0.014 atm
B) 0.037 atm
C) 0.456 atm
D) 0.01 atm
E) 11.2 atm
E) 11.2 atm
π = iMRT
For the first-order decomposition of N2O5 at 340 K, where k = 5.8 × 10-3 s-1, calculate the original concentration if the concentration of N2O5 is 0.41 M after 209 seconds.
a. 0.12 M
b. 0.23 M
c. 0.72 M
d. 1.4 M
e. 1.7 M
Around 1.4M
*ln[A]t=-kt+ ln[A]o*
What is the pH of an aqueous solution of 0.30 M HF and 0.15 M F- ? (Ka of HF = 7.2 × 10-4) (Hint: Buffer Solution)
a. 1.83 b. 2.84 c. 3.14 d. 3.44 e. 10.86
b. 2.84
pH=pKa+ [conj base]/[acid]
The solubility product for chromium(III) fluoride is Ksp = 6.6 × 10-11. What is the molar solubility of chromium(III) fluoride? (Hint: Ksp=[as]a[bs]b)
A) 1.6 × 10-3M D) 2.2 × 10-3 M
B) 1.2 × 10-3 M E) 1.6 × 10-6 M
C) 6.6 × 10-11 M
B) 1.2 × 10-3 M
Al3+ is reduced to Al(s) at an electrode. If a current of 2.00 ampere is passed for 48 hours, what mass of aluminum is deposited at the electrode? Assume 100% current efficiency. (mol x = A*t/nF)
a. 3.58 g b. 32.2 g c. 48.3 g d. 96.6 g
e. 2.90 × 102 g
b. 32.2 g
Current: 2 Amp.
Time: 48 hours ->172800 seconds
F=96500 C/mol
n(electrons per mol of Al)=3
What is the largest organ in the human body?
The skin