(˚ˎ 。7
|、˜〵
じしˍ,)ノ
What are the intermolecular forces taking place between water and CO2?
-London dispersion forces
-dipole-induced dipole
What is the rate expression of the following reaction?
6CO2 + 6H2O → C6H12O6 + 6O2
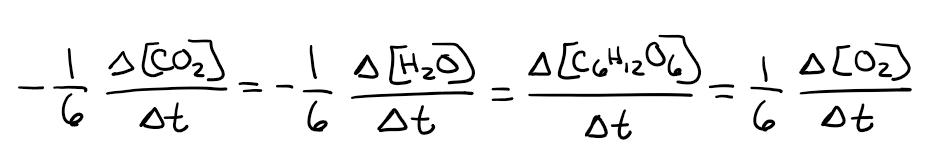
Given that the gibbs free energy is 550KJ/mol and the entropy is 3.4 J/mol*K, what is the enthalpy of the reaction at 25°C ?
ΔH ≈ 551 kJ/mol (at 25 °C)
What is the molality (m) of a solution made of 86.0 g of NaCl in 240 mL of DMSO? density of DMSO = 1.1 g/mL
5.57m
What type of crystal would a salt be called?
A) metallic crystal
B) covalent crystal
C) ionic crystal
D) ur mom
C) ionic crystal
Based on the cell diagram, what is getting reduced in the voltaic cell?
Cu(s)| Cu 2+(aq) ||Ag + (aq)| Ag(s)
Ag (silver)
What is the pOH of a solution with 0.4M of HNO3?
pOH=13.60
Is the reaction below exothermic or endothermic?
A(s) +B(g) + ΔH --> C(g)+ D(g)
endothermic
Which reaction would result in the highest entropy?
A) 2H2O(l)→2H2(g)+ O2(g)
B) CaCO3(s)→ CaO(s)+CO2(g)
C) CO2(g)+2H2(g)→ CH4(g)+2H2O(l)
D) N2(g)+3H2(g)→2NH3(g)
A) 2H2O(l)→2H2(g)+ O2(g)
Which salt is the most basic when dissociated in water?
A) NaBr
B) NH4NO3
C) LiSO3
D) KCl
C) LiSO3
Suppose a buffer solution is composed of 0.65M potassium formate (HCOOK) and 0.80M formic acid (HCOOH), pKa=3.70 for HCOOH. Find the pH of the buffer.
pH=3.61
What is the activation energy (Ea) of a process at 25°C where the rate constant = 0.65 s-1, and collision frequency
factor (A) = 80,000 s-1?
29.0 kJ/mol.
k=Ae-Ea/RT
What is the order of the reaction:
A+2B --> C +3D
rate= k [A]2[B]0
2nd order
Calculate the Ecell of the following reaction:
Mn(s)+ 2Ag(aq)--> Mn2+(aq) + 2Ag(s)
1.98V
Based on the following titration curve, what is the titrant?
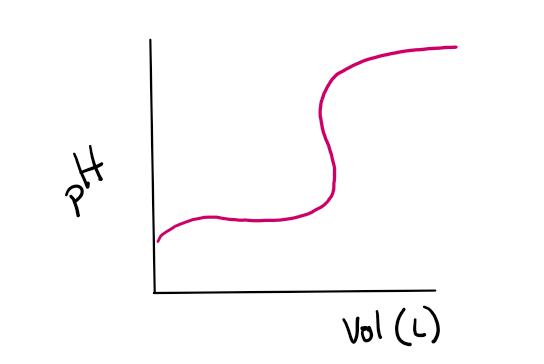
A) Acidic
B) Basic
C) Not enough information
D) huh?
B) Basic
Identify the conjugate base of HPO42- in the reaction
HCO3- + HPO42- --> H2CO3 + PO43-
A) H2O
B)HCO3-
C)H2CO3
D) PO43-
E) none of these
D) PO43-
What is the Keq expression of the following reaction?
C3H8(g)+ 5O2(g) → 3CO2(g) + 4H2O(g)
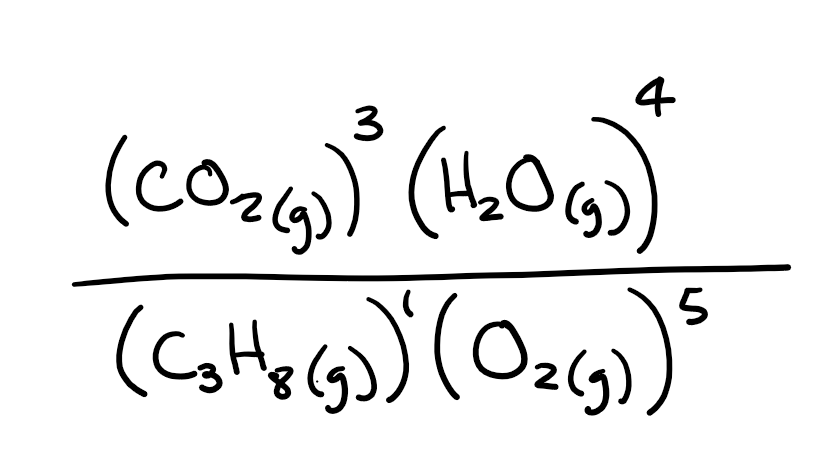
What intermolecular forces are taking place between ammonia (NH3) and sodium (Na)
- london dispersion ofrces
- ion - dipole
What is the 2nd law of thermodynamics?
A) energy can neither be created nor destroyed
B) the entropy of a perfect crystal at absolute zero Kelvin (0 K) is zero
C) entropy of the universe, as an isolated system, will always increase over time
D) an object will not change its motion unless a force acts on it
C) entropy of the universe, as an isolated system, will always increase over time
You titrate 6.0 mL of HF 0.3M with 18.0 mL of NaOH 0.1 M. What is the pH of the resulting solution?
HF Ka=7.2*10-4
pH=8.01