Carlos is developing a new chocolate chip recipe. While testing recipes out, he notices that when he adds less and less flour to the cookie dough, the cookies get wider and spread out on the pan. What is the dependent variable in Carlos’ experiment?
a) Type of flour used
b) Amount of chocolate chips added
c) Amount of flour added
d) Size of baked cookies
ANSWER: d Size of baked cookies
Remember that the dependent variable depends on the independent variable. In this problem, we see that the width (a.k.a. the “size”) of each cookie depends on how much flour Carlos puts in the dough.
Heat will flow from a material at a ____ temperature to one that is at a ____ temperature.
a) lower, higher
b) constant, higher
c) higher, lower
d) constant, constant
ANSWER: c
Heat will flow from a material at a higher temperature to one that is at a lower temperature.
pg 40
Which one of the following is NOT a solution?
- Cup of coffee
- Glass of apple juice
- Seawater
- Glass of milk
ANSWER: 4. Glass of milk
What is milk?
A colloid
Which one of the following elements is a non-metal?
- Gold
- Carbon
- Zinc
- Iron
ANSWER: 2) carbon
A solution is said to be ________________ when no more solute will dissolve in it.
- saturated
- diluted
- unsaturated
- suspended
ANSWER: 1) saturated
A local gardening club has started a new compost bin, and they begin by putting a small number of worms in the starter soil. Every week, they open the bin and count how many worms are working through the compost. Using this information and the graph below, choose which measurement is the independent variable.
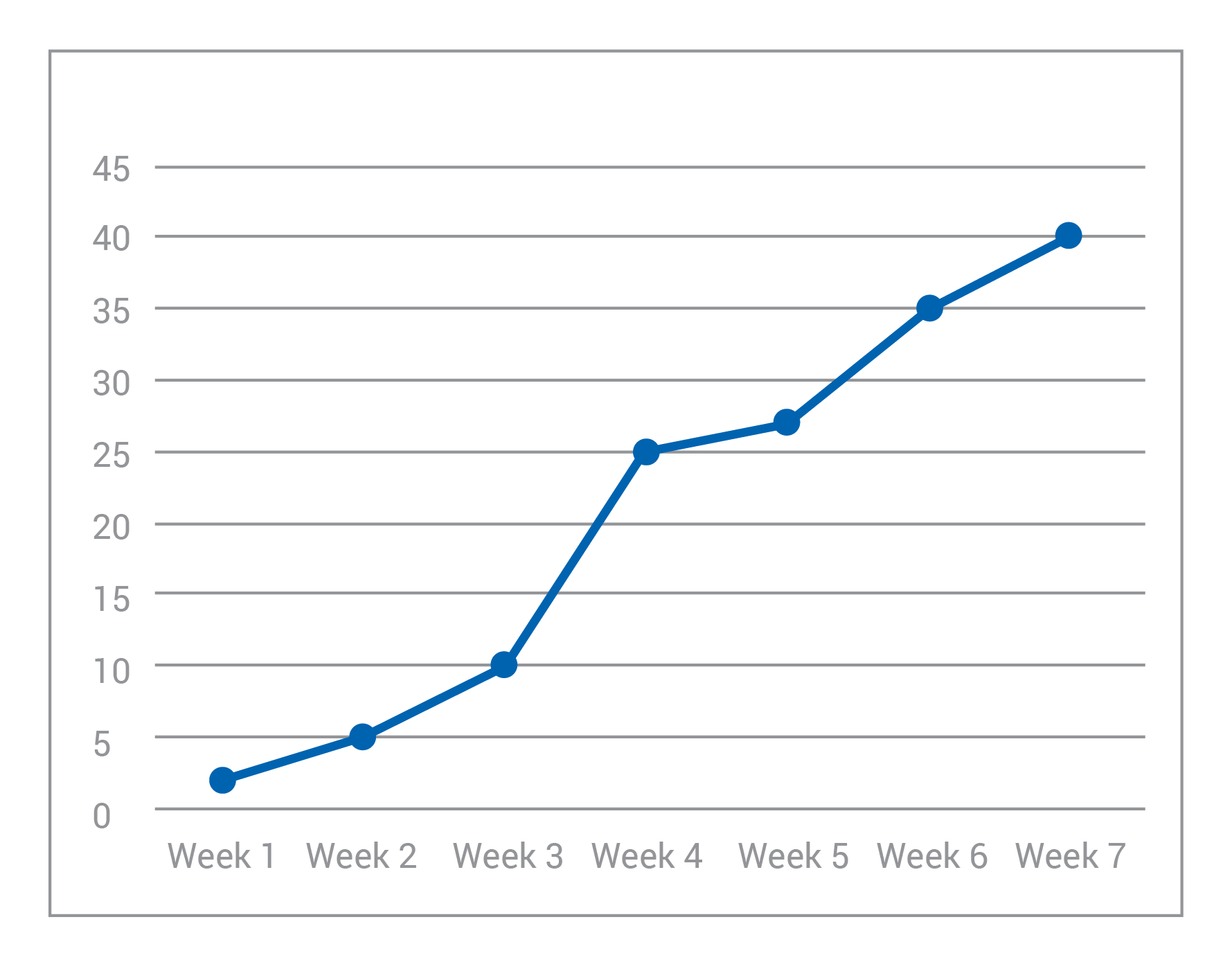
a) Time (weeks passed)
b) Length of worms in the compost bin
c) Amount of worms in the compost bin
d) Amount of compost generated
ANSWER: a) Time (weeks passed)
The correct answer is A. While we may not be making time go on or changing time itself, we see that the amount of worms grows as time goes on. Hence: the worm population depends on how much time has passed.
*Also, even if we didn’t fully understand what was going on in this experiment, we can count on two “cheat cards:” time is almost always an independent variable; and we usually put the independent variable on the x-axis and the dependent variable on the y-axis when graphing experiment results.
When copper sulfate crystals are dissolved in water, the mixture is known as
- an emulsion
- a solution
- a solvent
- a solute
ANSWER: 2. A solution
Over the past few weeks, Briana has been conducting an experiment to see what type of bread grows the most mold within a given period of time. She’s chosen 6 different types of bread, and as time has passed, she’s measured the areas of each mold patch that’s grown on the respective bread slices. The graph below shows her results:
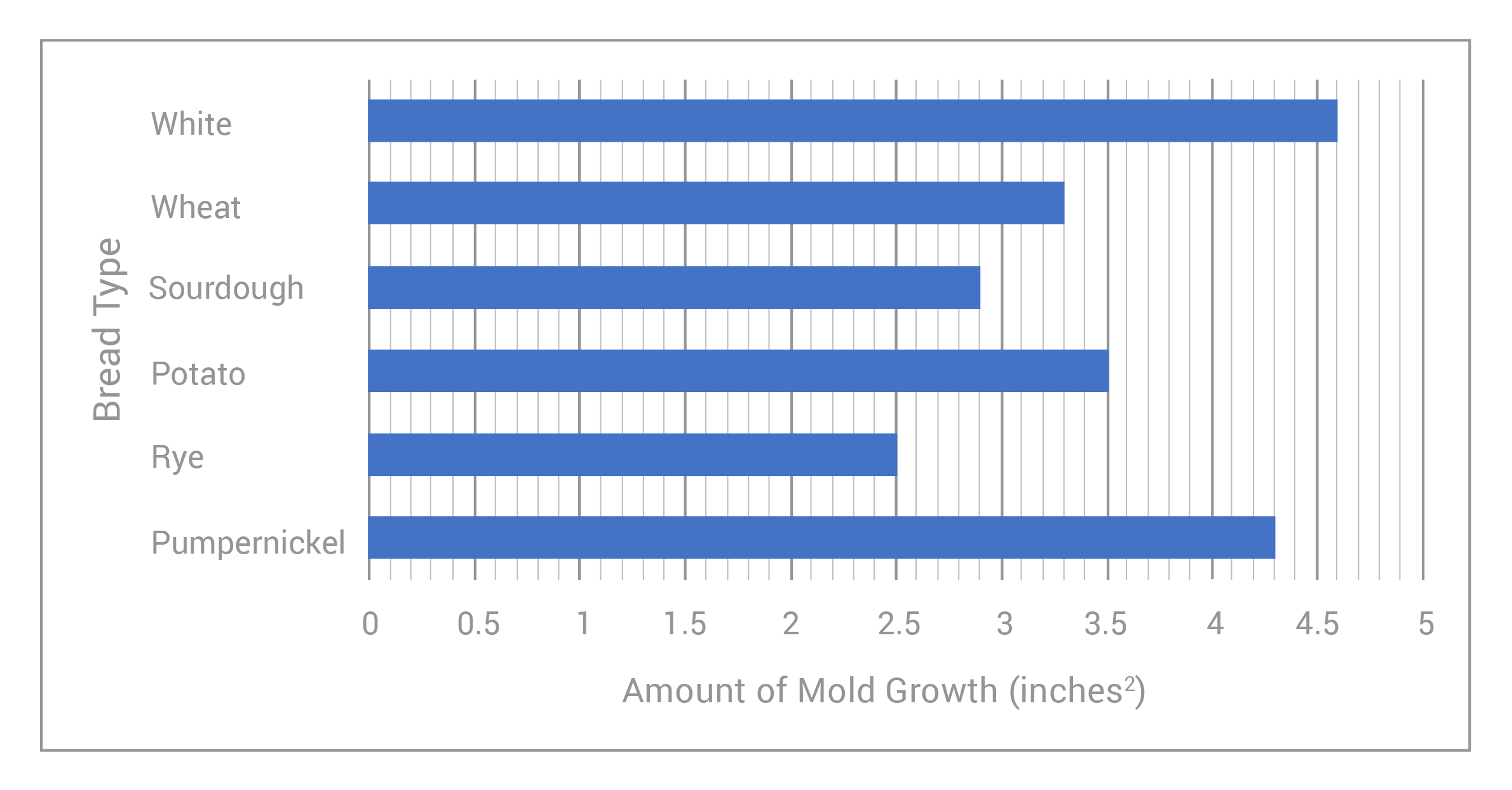
What is the independent variable in Briana’s experiment?
a) Time (weeks passed)
b) Bread type
c) Amount of mold growth (inches2)
d) Area of bread slices (inches2)
ANSWER: b, Bread Type
The correct answer is B.
While it may be tempting to choose Choice A, time is actually a controlled variable (the “control”) in this experiment. We observed a phenomenon over a pre-set, controlled period of time. Really, we need to ask ourselves, “What is Briana changing?” (this will always be our independent variable) and “What is she measuring/observing?” Of course, as Briana has changed the type of bread, she’s observed a change in the amount of mold grown on each slice; so it’s clear that our independent variable is the bread type!
*Additionally, Briana has noticed that the amount of mold growth on each slice is different for each type of bread. Hence, it’s safe to say that the ‘amount of mold growth’ depends on the ‘bread type.’ Meaning: the amount of mold growth is Briana’s dependent variable!
**One final note: notice that the dependent variable sits on the x-axis of this graph, and the independent variable sits on the y-axis. This is different from what we usually see! So, it’s important that we take the “x-axis=independent variable and y-axis=dependent variable” trick with a grain of salt. Always read the question and the graph labels carefully!
In the Periodic Table of the elements, what information is given by the number above each chemical symbol?

- Its group number
- The atomic number of the element
- Its period
- The mass number of the element
ANSWER: 2. The atomic number of the element
What is the atomic number?
# of protons & electrons
What do the elements of group II (2) of the periodic table have in common?
- Same atomic number
- Nothing in common
- Similar chemical properties
- Same mass number
ANSWER: 3,
- Similar chemical properties
Isotopes are atoms of the same element with different numbers of _______.
a) protons
b) electrons
c) atomic weight
d) neutrons
ANSWER: d, neutrons
pg 45
What is the trend for group VII (7) elements as they go down the group?

- There is no change in their reactivity going down the group.
- They become more soluble in water as they go down the group.
- They increase in order of reactivity.
- They decrease in order of reactivity.
ANSWER: 4.
- They decrease in order of reactivity.
- The most reactive is at the top of the group!
There is a change in their reactivity going down the group.
A __________is a device used to measure the pressure of a fluid
a) thermometer
b) tripple beam balance
c) bunsen burner
d) manometer
ANSWER: d, Manometer
pg. 108
Manometers often measure pressure based on differences in the height (H) and position of a column of liquid in a “U” tube
Barometer: a type of manometer used to measure atmospheric pressure.

On the image above, what color represents the chemical's reactivity rating?
a) blue
b) yellow
c) red
d) white
• Reactivity rating: indicates the likelihood of the substance to release energy by chemical reaction or explosion.
pg 111
Blue: health
Red: flammability
White: special warning
"Happy Flamingos Run Sideways"
Which of the following elements is a metal?

- Neon
- Sodium
- Carbon
- Phosphorous
ANSWER: 2, sodium
A compound is a substance that:
- is made up of only element.
- contains two or more elements physically combined together in a fixed ratio.
- is made up of two or more elements chemically joined together.
- contains only metallic elements chemically joined together.
ANSWER: c, is made up of two or more elements chemically joined together.
A flask is a:
a). a small cylindrical glass tube that has a rounded, u-shaped bottom
b) a glass container with a thin “neck” that widens to a round or cone shaped base.
c) a wide, open container with a flat bottom made of glass or plastic.
d) : a tall, cylindrical container used to measure the volume of a liquid.
ANSWER: b
A mixture of a liquid and a fine insoluble solid is known as:
- a solvent
- a suspension
- a solute
- a solution
ANSWER: b, a suspension
Example: sand and water
Fill in the blanks:
A dilute solution is one that contains a lot of ______
and little _________.
- solvent, solid
- solute, solvent
- solvent, solute
- solute, suspension
ANSWER: 3, solvent, solute
The elements in group I of the periodic table have similar chemical properties because:
- they are all metals.
- they all have a single electron in their outer shell.
- they all look similar.
- they all react with water.
ANSWER: b, they all have a single electron in their outer shell
An Irish scientist who is now considered the father of chemistry proposed the first modern definition of an element. This scientist was

- Isaac Newton
- Robert Boyle
- George Boole
- Robert Kane
ANSWER: 2. Robert Boyle
The minimum amount of energy needed before reactants can form a product is called:
- reaction energy
- activation energy
- nuclear energy
- exothermic energy
ANSWER: 2, activation energy
pg. 86
Complete the following sentence:
In any chemical reaction, the total mass of the reactants is always equal to the mass of the ___________.- molecules
- particles
- processes
- products
ANSWER: 4, products
For example, the carbon atom in coal becomes carbon dioxide when it is burned. The carbon atom changes from a solid structure to a gas but its mass does not change.
Adding sugar to a cup of tea is a physical change because

- the tea tastes sweeter
- there is no change in color
- there is no change in temperature
- it is possible to separate the sugar from the tea
ANSWER: 4,
- it is possible to separate the sugar from the tea
A process that involves heat being taken in is called
- an endothermic process
- a physical change
- an exothermic process
- a chemical reaction
ANSWER: 1. an endothermic process
endo-internal, within