Can you eat food or drink water/dutch/coffee in the lab?
What is no
What are the charges for the following atoms...
- Proton
- Neutron
- Electrion
What is...
- Proton = Positive
- Neutron = Neutral
- Electron = Negative
What are the rows in the periodic table called?
What is Periods
What type of bond involves the sharing of electrons?
What is Covalent Bond
A state of matter that does not have definite volume and no definite shape?
What is gas
What is the chemical formula for dihydrogen monoxide?
What is H2O
Is it okay to talk other members within your group?
What is yes
An element has 32 protons and 34 neutrons. How electrons does this element have?
What is 32 electrons
What element is found in Period 5 and Group 13(3A)?
What is Indium
What bond involves the transfer of electrons?
What is Ionic Bond
What is the name of this phase change...
Solid <-------- Gas
(Gas goes directly to solid)
What is Deposition
What is the chemical name for NaCl?
What is Sodium Chloride
If you break a beaker, what do you do?
What is Tell your instructor immediately
What is the mass number of Bromine-81?
What is 81
Which of these elements have the greatest atomic radius?
K, Y, Co, N
What is K
What kind of ion is formed when a neutral calcium turns into a calcium ion with a positive charge of two?
Ca --------> Ca2+
What is cation
If I pour a brand new container of salt entirely into a beaker that is half full with water, I have a salt solution that is...
What is Supersaturated
What is the chemical name for P2O4?
What is Diphosphorus tetraoxide
What two items do you bring to the lab bench when starting lab?
If you draw the Bohr's model for Chlorine (Cl), how many electrons will the last orbital have?
What is 7 electrons
What group of elements are lined up on the staircase in the periodic table? 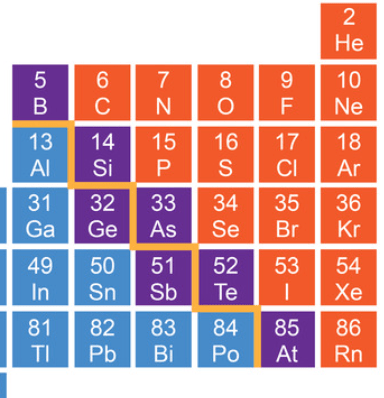
What is metalloids
Draw the electron dot structure (lewis dot structure) for N3-
What is...
If I have a glass of freshly squeezed orange juice, what kind of solution is this?
What is a Homogenous mixture
What is the chemical formula for Ammonium chloride?
What is NH4Cl
If you have any leftover chemicals, what should you do?
What is Dispose chemicals properly into designated container
What is the electron configuration for Aluminum (Al)?
What is...
1s22s22p63s23p1
or
[Ne]3s23p1
What 3 trend occurs when you go up and to the right on periodic table?
What is...
Electronegativity
Ionization Energy
Electron Affinity
Draw the Electron Dot Structure (Lewis Dot Structure) for O2?
What is...
What phase will carbon dioxide (CO2) be at 30 atm of pressure and -50˚C? 
What is a liquid
What is the formula for Iron(III) Oxide?
What is Fe2O3
What should you do if someone is hurt in the lab?
A. Run out of the class with the injured student to take them to the nurse.
B. Yell out "Code One" to get the instructor's attention.
C. It was just a small cut... best wrap it up and don't bother anyone.
D. Send another student to find the instructor and bring attention to the injury.
What is Yell out "Code One" to get the instructor's attention.
This imagine show a violation to what principle?
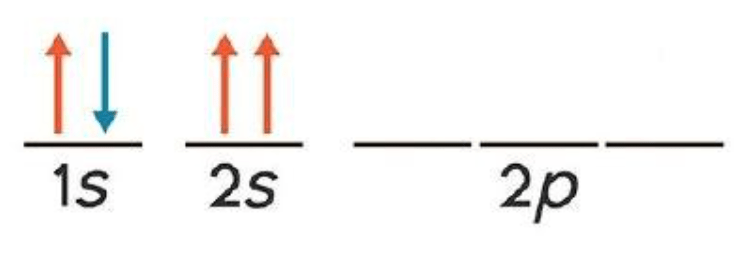
What is the Pauli exclusion principle
Which elements have similar properties?
O and N
C and Si
K and Ca
Ne and Li
What is C and Si
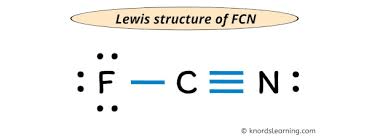
Draw the Electron Dot Structure (Lewis Dot Structure) for FCN
what is
How many grams can I dissolve of K2Cr2O7 at 90˚C to have a saturated solution?
What is 70 grams
What is this name for this compound?
Mn(CO3)2
What is Mangenese(IV) Carbonate