What is the name of FeO2?
Iron (IV) Oxide
What is the difference between polar and nonpolar bonds?
A covalent bond is nonpolar if the difference in electronegativity between the 2 atoms ranges from 0 to 0.4. A nonpolar covalent bond = the bonding electrons are shared equally between the two atoms.
If the electronegativity difference between 2 atoms is between 0.4 and 1.7 then it is a polar covalent bond. A polar covalent bond = Unequal sharing of bonding electrons
How many atoms of carbon are in 76.9 mol of ethane (C2H6) at STP? Must show work.
9.26x1025 atoms of carbon
What is the difference between actual yield and theoretical yield? What is the formula for percent yield?
Actual yield- What you ACTUALLY mde. The amount of product you produce in the lab when chemical are mixed.
2. Theoretical yield- What SHOULD BE made. The maximum amount of product that could be formed from given amounts of reactants. Determined from calculations using the balanced reaction.
3. Percent yield – the ratio of the actual yield to the theoretical yield.
Percent yield = (actual yield/theoretical yield) x 100
What is enthalpy? If the enthalpy is negative, what does it tell you about the system? If the enthalpy is positive, what does it tell you about the system?
What is the name of PCl5?
Phosphorus Pentachloride
Draw the lewis structure of PCl3.
Determine the shape name and bond angle.
Determine if bonds are polar or nonpolar.
Determine if entire molecule is polar or nonpolar.
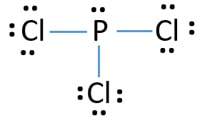
3d shape

Trigonal Pyramidal
107 bond angle
P-Cl = 3.16 - 2.19 = 0.97 so all bonds are polar.
Molecule is always polar due to lone pairs.
Determine the products of the following reactants:
MgO + NaBr -->
MgO + NaBr --> MgBr2 + Na2O
When iron (II) chloride reacts with sodium phosphate, iron (II) phosphate and sodium chloride are formed.
Balance the following equation:
______ FeCl2 + ______ Na3(PO4) → ______Fe3(PO4)2 + ______ NaCl
2. 23 grams of iron (II) chloride reacts with 41 grams of sodium phosphate. How much sodium chloride can be formed?
3. What is the limiting reactant for this reaction based on your work? __________________
What is the excess reactant? __________________
How much excess reactant is leftover after the reaction is completed?
3 FeCl2 + 2 Na3(PO4) → 1 Fe3(PO4)2 + 6 NaCl
21.21 g NaCl
Limiting = FeCl2
Excess = Na3(PO4)
Excess leftover = 21.17 g
What is the difference between kinetic energy and temperature?
Kinetic energy is the energy of motion.
Temperature is a measure of average kinetic energy (KE) or average motion of particles NOT the total kinetic energy.
What is the formula for Zinc (III) Sulfide?
Zn2S3
Draw the lewis structure of Zinc (III) Bromide.
Determine the shape name and bond angle.
No shape or bond angle because the molecule is ionic. It has a crystal structure. Ionic molecules do not follow VSEPR shapes.
Balance the following reaction:
C2H6 + O2 → CO2 + H2O
2 C2H6 + 7 O2 → 4 CO2 + 6 H2O
In the lab, 50.2 g of water is produced when 276 g of NH4NO3 decomposes. Calculate the percent yield of H2O.
_____ NH4NO3 → _____ N2O + _____ H2O
1 NH4NO3 → 1 N2O + 2 H2O
Actual yield = 50.2
Theoretical yield = 124.13 g
Percent yield = 40.44%
Draw a fully labeled energy diagram for endothermic reactions.
What is the formula for Silicon Dioxide?
SiO2
Do metals tend to be more of less electronegative than nonmetals? Why?
Metals
They tend to be cations
Have a positive charge
Want to LOSE electrons
So that means they will not attract electrons strongly when bonded to nonmetals
Not very electronegative
Nonmetals
- They tend to be anions
- Have a negative charge
- Want to GAIN electrons
- So that means they will attract electrons strongly when bonded to metals
- Very electronegative
Determine the products of the following reactants:
ZnCl2 + O2 -->
ZnCl2 + O2 --> ZnO + Cl2
Translate the following reaction into a sentence.
___ H2(g) + ___ NO(g) → ___ H2O(l) + ___ N2(g)
Hydrogen gas reacts with nitrogen monoxide gas to yield liquid dihydrogen monoxide and nitrogen gas.
Draw a fully labeled energy diagram for exothermic reactions.