What are atoms made of?
Protons, Neutrons and Electrons
What are the two types of pure substances?
Elements and Compounds
Which of these is a mixture of compounds?

C
What do the letters represent in chemical formulas? (Example: The H and O in H2O)
Elements
What do we call the things on the left side of an equation?
Reactants
What causes a substance to change states of matter?
adding or taking away thermal energy
What are the charges of these particles?
electrons = negative
protons = positive
neutrons = neutral
These can be found on the periodic table and are a substance that cannot be broken down into any other substance.
Elements
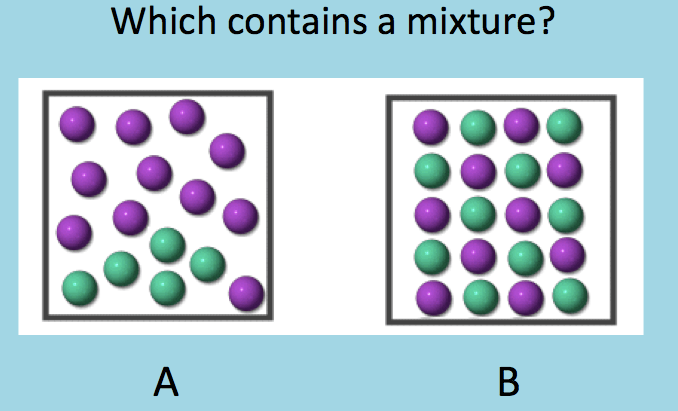
A & B
The number of atoms.
What do we call the things on the right side of an equation?
Products
Which state of matter has the least energy
Where are neutrons located in the atom?
In the nucleus
Which of these is a pure substance?

Figure 1
Give me an example of a mixture.
Various
What do the coefficients represent in a chemical formula? (Example: the 3 in 3H2O)
The number of molecules.
Is this equation balanced or unbalanced?
H2 + N2 --> NH3
Unbalanced
Which state of matter is found between the boiling point and the melting point of a substance?
liquid
How do we get the atomic mass?
The number of protons + the number of neutrons
This is a pure substance that is a combination of two or more elements bonded together.
Compound
True or False:
Substances in a mixture are combined physically not chemically.
True
How many Oxygen atoms are in this formula?
3H2O?
3
Is the following equation balanced or unbalanced?
C + 2H2 --> CH4
Balanced

What state of matter is point S?
liquid
What determines the identity of an element?
Which of these are pure substances?

A & B
What are the two types of mixtures?
Homogenous and Heterogenous
How many iron atoms are in this formula?
2Fe2O3
4
Balance the following equation:
Na3PO4 + KOH --> NaOH + K3PO4
Na3PO4 + 3KOH --> 3NaOH + K3PO4

What state of matter is point R?
Gas