A change that does not change the substance
What is a physical change
What are the two types of pure substances?
Elements and Compounds
Which of these is a mixture of compounds?

C
What do the letters represent in chemical formulas? (Example: The H and O in H2O)
Elements
What do we call the things on the left side of an equation?
Reactants
What causes a substance to change states of matter?
adding or taking away thermal energy
A change that alters the substance or forms a new substance
What is a chemical change?
These can be found on the periodic table and are a substance that cannot be broken down into any other substance.
Elements
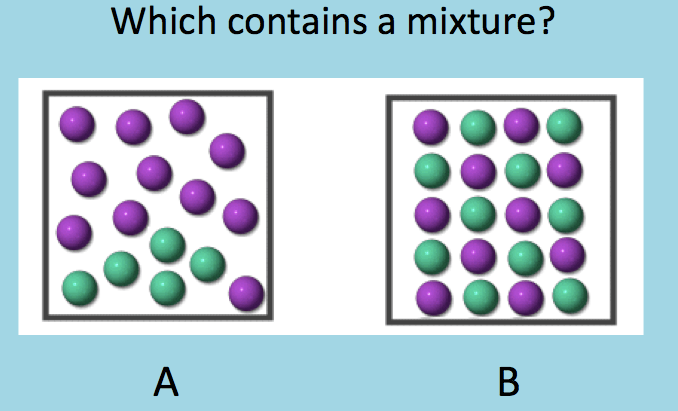
A & B
What is the subscript in H2O
What do we call the things on the right side of an equation?
Products
Which state of matter has the least energy
Burning: Physical or chemical change
What is a chemical change
Which of these is a pure substance?

Figure 1
Give me an example of a mixture.
Various
What is the coefficient in 3H2O
What is 3
Is this equation balanced or unbalanced?
H2 + N2 --> NH3
Unbalanced
Which state of matter is found between the boiling point and the melting point of a substance?
liquid
Melting: Physical or chemical change
What is a physical change
This is a pure substance that is a combination of two or more elements bonded together.
Compound
True or False:
Substances in a mixture are combined physically not chemically.
True
How many Oxygen atoms are in this formula?
3H2O?
3
Is the following equation balanced or unbalanced?
C + 2H2 --> CH4
Balanced

What state of matter is point S?
liquid
The 4 pieces of evidence that a chemical change has occurred
1) change in color
2) formation of a precipitate
3) gas production
4) change in energy or temperature
Which of these are pure substances?

A & B
What are the two types of mixtures?
Homogenous and Heterogenous
How many iron atoms are in this formula?
2Fe2O3
4
Balanced or unbalanced:
Na3PO4 + KOH --> NaOH + K3PO4
What is unbalanced

What state of matter is point R?
Gas