Does hyperkalemia cause muscles to become tight or contracted or function to be low and slow?
Tight and Contracted
Heart= ST elevation and peaked T waves
Severe Vfib or Cardiac standstill, Hypotension and Bradycardia
GI= diarrhea, hyperactive bowel
Neuromuscular= Paralysis in Extremities, Increased deep tendon reflex, and profound muscle weakness
In HYPOkalemia muscle function is "low and slow"i.e. hypoactive bowel sounds (constipation), flaccid paralysis (paralysis in the entire limb), alow-absent DTR
What natural process does your body (cells) use to maintain homeostasis in fluids?
Osmosis
Can someone describe what osmosis is vs diffusion and active transport?
What are the normal PaCO2, pH and HCO3 (or bicarbonate) levels in the body?
Challenge yourself:
Can you draw arrows describing their relationship with each other?

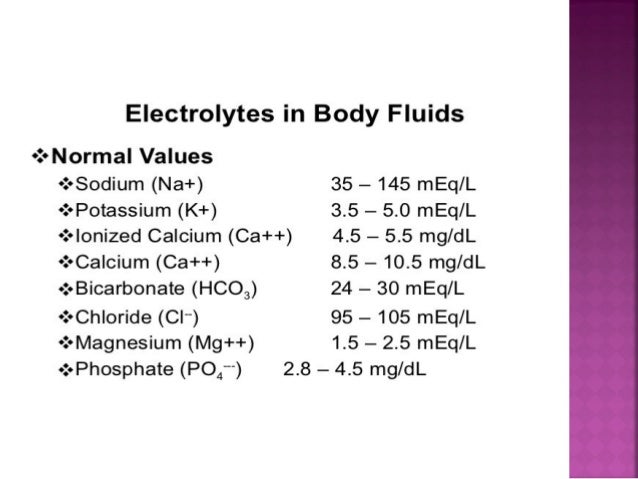
BONUS POINTS: For 100 points tell me the normal amount of any electrolyte found in the human body.
Get an additional 100 points for each additional electrolyte you know the values of!
DAILY DOUBLE
The excessive sweat and decreased salt and fluid absorption characteristic of Cystic Fibrosis cause Chloride Depletion.
Knowing that the electrolyte abnormality of decreased chloride can easily occur with CF patients. What signs/ symptoms should you look out for?
nausea, vomiting
be aware that excessive sweat can exacerbate chloride deficiency and make patient more uncomfortable. Therefore try to prevent extra stress, added heat, vigorous physical exercise, etc
What electrolyte imbalance would be of most concern for a malnourished man who has been started on TPN (total parental nutrition)?
Hypophosphetemia can occur if calories are started too aggressively.
Nursing Intervention: It is important to gradually introduce solution to avoid rapid shift of phosphorus into the cells.
Additional question: What kind of foods are high in phosphate?
Milk, Poultry, Liver etc
Name at least one isotonic, hypotonic and hypertonic fluid solution commonly used in the hospital setting.
Isotonic: 0.9% Saline, 5% Dextrose in Water, 5% Dextrose in Lactated Ringers (might be considered hypotonic too)
Hypotonic: 0.45% Saline, 0.225 Saline, and 0.33% Saline
Hypertonic: 3% Saline, 5% Saline, 5% Dextrose in 0.9 and 0.45% Saline, 5% Dextrose in LR, and 10% Dextrose in water
A nurse is caring for a client with a nasogastric tube that is attached to low suction. The nurse monitors the client closely for which acid-base disorder that is most likely to occur in this situation?
1. Metabolic acidosis
2. Metabolic alkalosis
3. Respiratory acidosis
4. Respiratory alkalosis
2. Metabolic alkalosis
How? Hydrogen ions may be lost by the kidneys or GI tract (this is why in respiratory acidosis/ alkalosis kidneys compensate)
What kind of fluid will typically be used in patients with Diabetic Keto-acidosis or hyperosmolar hyperglycemia (both cases in which the cell itself is very dehydrated)?
Hypotonic solutions!
Why is a nutritional deficit so prevalent with COPD patients?
The lung expansion causes the diaphragm not to work properly. Usually the diaphragm helps us effortlessly breath however when it is not functioning properly (such as the case in COPD) patients begin to use their accessory muscles. This increased effort just to breath depletes many calories and energy. Many patients may be too exhausted to eat on top of this.
Patient comes into hospital with symptoms of Polydyspia (excessive thirst), swollen tongue, edema and low-grade fever.
What Electrolyte Imbalance would you suspect?
Hypernatremia
Helpful hint: "Big and Bloated"
Other signs and symptoms include nausea and vomiting and increased muscle tone
HYPOnatremia= "Depressed and Deflated"
Neurological:
Seizures & Coma
Heart: tachycardia and weak and thready pulse lastly respiratory arrest
Hypochloremia is very similar only major difference hypo may cause fever
Oxygen and carbon dioxide are exchanged between pulmonary capillaries and alveoli.
What process does this illustrate?
Diffusion which is the tendency for a substance to move from a higher to lower concentration.
A client has the following lab values: a pH of 7.55, an HCO3- of 22 mm Hg, and a PCO2 of 30 mm Hg. What should the nurse do?
1. Perform Allen's test
2. Prepare the client for dialysis
3. Administer insulin as ordered
4. Encourage the client to slow down breathing
4. Encourage the client to slow down breathing
What two electrolytes always work inversely?
a) magnesium and calcium
b) calcium and phosphate
c) sodium and magnesium
d) chloride and sodium
b) calcium and phosphate
Therefore Hypocalcemia and Hyperphosphatemia show similar symptoms/ signs and vice versa!
These two electrolytes work inversely, what system in the body regulates them?
What hormone/ where?
For patients with emphysema what happens with the typical PaCO2 and PaO2 overtime?
PaCO2 increases greatly while O2 drops.
What kind of actions does the body take to reobtain homeostasis? What are the harmful effects?
1. Due to increased oxygen body begins to make more red blood cells= blood becomes thick= thats ineffective
2. Body reacts with increased pressure in the arteries due to shifting blood
3. This leads to pulmonary hypertension
4. Can cause eventual heart failure
(play RN Sarah 10:20 if you need additional information!)
Important to note: over administration of oxygen will lead to carbon dioxide narcosis and hypoxemia.
Patients has just arrived back on the unit following a thyroidectomy and reports tingling in the lips and fingers. She has an intermittent spasm in her hand and wrist and exhibits increased muscle tone.
What electrolyte imbalance is to blame?
Hypocalcemia
How else do we assess for Hypocalcemia= Chvotseks and Trousseu's sign!
What kind of fluid do you need to avoid at all cost when dealing with a patient with increased cranial pressure or bad burns?
Hypotonic Solutions. This is because it may lead to hypovolemic shock.
The nurse is told that the blood gas results indicate a pH of 7.55 and a PCO2 of 30 mm Hg. The nurse determines that these results indicate:
1. Metabolic acidosis
2. Metabolic alkalosis
3. Respiratory acidosis
4. Respiratory alkalosis
4. Respiratory alkalosis
Why do people sometimes use a paper-bag when someone is having a panic attack? Describe this in terms of acid and base.
Breathing too fast, as often happens with anxiety and panic attacks, can lead to respiratory alkalosis. Therefore breathing into a paper bag (an older technique) can help someone by getting back some of the lost CO2.
How do respiratory regulations differ in someone with COPD?
CO2 is always high causing there respiratory medulla to be insensitive to these changes thus making it harder for them to regulate acid-base imbalances.
What kind of chest does a patient with severe emphysema typically have?
barrel or pigeon chest?
Those with emphysema are Barrel Chested
(remind yourself of when you measured AP diameter in Skills)
Typically with barrel-chested patients the anterior is equal to the posterior measurements of the chest
A patient is admitted to the unit with hypotension, bradycardia, Decreased DTR, and SHALLOW respirations.
What electrolyte imbalance is suspected that is often identified by shallow breaths?
Hypermagnesia
Helpful Hint="Calm and Quiet"
Additional symptoms: hypoactive bowels
For Magnesium:
Helpful hint "Law and Order"in muscles: Heart, uterus and DTR
Without enough magnesium or the sheriff everything is WILD. Therefore Hypomagnesia causes
Heart: Tachycardia, V fib, ST depression, T wave inversion, Torsades de pointes
GI=diarrhea
Increased DTR and eyes go CRAZY= nystagmus; which is abnormal eye movement
Magnesium is required for calcium and vitamin D absorption. Therefore, think about foods high in magnesium you may want to give your patient.
Water and electrolytes move from capillary bed to interstitial fluid. What causes this to occur?
Hydrostatic pressure resulting from the pumping of the heart
What important roles do the kidneys play in metabolic acidosis?
The kidneys excrete hydrogen ions and conserve bicarbonate ions to restore kidney balance.
What causes the release of anti-diuretic hormone (ADH)?
Increased serum sodium levels
THINK BACK to learning about the kidneys!
Which picture more closely resembles someone who has COPD?
Look at Julia as she displays three different photos
The tripod position
This photo displays the hunched over back of a typical COPD patient using his/her accessory muscles to breath