Name the 3 subatomic particles that make up atoms.
Protons, Neutrons, Electrons
What is the atomic symbol, number, and mass of the element shown below?

C
6
12.011
Protons = +
Neutrons = No charge
Electrons = -
What are valence electrons? And how do they trend on the periodic table?
Electrons on the last shell of an atom.
They are the same down every column
What is the term for when bears go to sleep during the winter?
Hibernation
Where are the 3 subatomic particles located in an atom?
Protons and neutrons = nucleus
Electrons = outside on the shells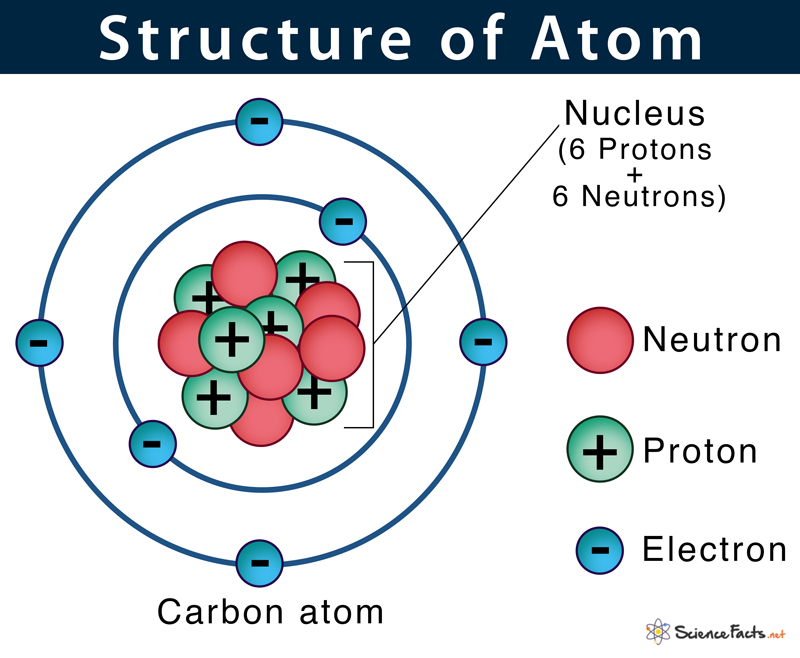
What does the atomic number tell you about an element?
How many protons and electrons an element has
Which subatomic particle has virtually no mass?
What is the term for when an atom is likely to LOSE electrons?
Ionization energy
How many planets are there in our solar system? (Not including Pluto)
8
The number of ________ and _________ are equal.
Protons and electrons
What does the row an element is in tell you about how you should draw it?
It tells you how many shells the atom has
What is the purpose of protons?
The number of protons determines the element
Which element below would be most likely to GAIN electrons?
Argon (Ar)
Magnesium (Mg)
What kind of water is MOST of the water on Earth?
Salt-water
How do you find the number of neutrons in an atom?
Atomic mass - # of protons
*don't forget to round the mass!*
How many periods are there on the periodic table?
7
What is the purpose of electrons?
Create chemical bonds between other elements
Describe the how the atomic radius changes on the periodic table.
Increases right to left, and top down.
Name one organ system in your body.
Answers vary
How many electrons can go on each shell? Shells 1, 2, and 3.
2 on shell 1
8 on the rest
What family is the element Krypton in?
Noble Gases
How many valence electrons are on this atom?

One
Name the element with the highest atomic radius according to the trend.
Francium
How do you calculate the density of an object?
Mass / volume