Gas particles are considered to have perfectly elastic _______________
collisions
If a sample of gas at a certain volume and pressure is compressed, the pressure will ______________
increase
What unit must be used for volume in Ideal Gas Law?
Liters
What are the conditions of STP?
1 atm and 0oC
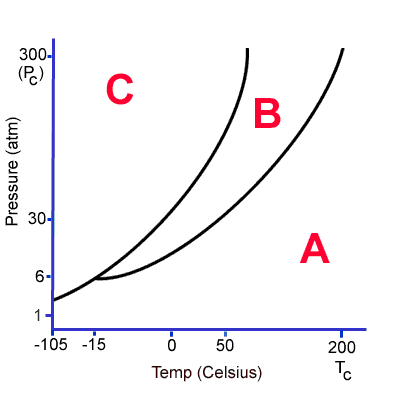
Region A is a ______
Gas
Gas particles are in constant, random, ________
motion
A balloon that is submerged in liquid nitrogen (very cold!) will __________ its volume
decrease
What is the value of R?
0.0821
A mixture of nitrogen and oxygen gas has a pressure of 1 atm. If the partial pressure of nitrogen is 0.75 atm, what is the partial pressure of oxygen?
0.25 atm

Region C is a _________
Solid
Pressure
increase
What number must be added to oC in order to convert to Kelvin?
273
A gas at 4 atm and 27o C is cooled to -10oC. What is the new pressure?
3.5 atm

The phase change that occurs when moving from B to C is _________
Freezing
____________ is defined as the average kinetic energy of a collection of particles
Temperature
A balloon with a volume of 300 mL at 250 K is heated to 350 K- what is the new volume of the balloon?
420 mL
At what temperature will 0.654 moles of neon gas occupy 12.30 liters at 1.95 atmospheres?
447 K
A gas has a volume of 800.0 mL at −23.0 °C and 3 atm What would the volume of the gas be at 227.0 °C and 6 atm of pressure?
800 mL

The phase change that occurs when moving from B to A is _____________
Vaporization
Increasing the temperature of a gas in a fixed container will increase the pressure because it increases the ____________ of the particles
speed
A piston has an initial volume of 200 mL and a pressure of 1.5 atm. The plunger is lifted so the volume increases to 1.2 L. What is the pressure inside the piston?
0.25 atm
What pressure is produced by 2 moles of CO2 occupying a 3 L container at 25oC?
16.3 atm
A sample of argon gas at STP occupies 56.2 liters. Determine the number of moles of argon.
2.5 moles

The phase change that occurs when moving from A to C is ___________
Deposition