The average amount of kinetic energy of all the particles together is measured with...(page 7)
temperature
gas particles are very ___________ and gases are full of ____________ space. (page 7)
small, empty
100oC = __________ K
373 K
Dalton's Law: There are 4 gases in a container. The total pressure of the container is 27 atm. The partial pressure of oxygen is 5 atm, the partial pressure of nitrogen is 9 atm, and the partial pressure of hydrogen is 8.5 atm. What is the partial pressure of argon gas?
4.5 atm
What type of relationship do volume and temperature have?
direct
Draw a graph for Charles' Law.
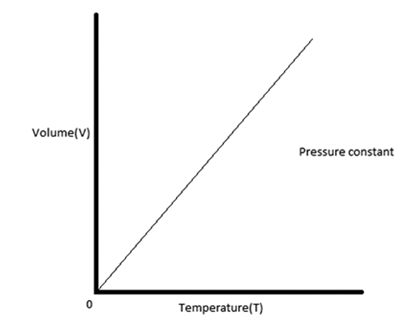
The amount of space all the particles take up (how big the container is) page 7
volume
The average kinetic energy of a gas is equal to the __________ of the gas. (page 7)
temperature
67oC = ____________ K
340 K
Boyle's Law: If the pressure is increased from 100 kPa to 200 kPa, what will the volume be if it starts at 50 mL?
25 mL
What type of relationship do pressure and temperature have?
direct
Draw a graph for Gay-Lussac's law.

The amount of force being applied to a given area. (how hard the particles collide with the walls of the container) page 7
pressure
When gas particles collide against the walls of the container or against each other, _________ is exerted. (page 7)
pressure
500 K = ___________oC
227oC
Gay-Lussac's Law: If the temperature of a 90 kPa gas is 300 K and then it decreases to 100 K, what is the new pressure?
30 kPa
What type of relationship do pressure and volume have?
inverse or indirect
Draw a graph for Boyle's Law.

How many particles there are in the container if they were counted one by one. (page 7)
quantity (number of moles)
Gas particles are in constant, rapid, random, straight-line __________ (page 7)
motion
1038 K = _____________ oC
765oC
Charles' Law: If you increase the volume from 100 mL to 200 mL, what will the new temperature of a 325 K gas be?
650 K
If volume increases by 4, what will pressure do?
decrease by 4
CHOOSE ALL THAT APPLY: Which of these graphs DO NOT represent one of our 3 gas laws?

2 & 4
temperature is a measure of this. (page 7)
kinetic energy
gas particles do not __________ or ___________ each other. (page 7)
attract, repel
Convert 492oC to K, divide by 2, then convert back to oC.
109.5
Ideal Gas Law: How many moles of gas occupy 20 L at 303 K and 4 atm? (R = 0.0821)
N = PV / RT
3.22 mol
If pressure increases by 2, what will temperature do?
increase by 2
What will a graph that represents this data look like?

curved